1
Question
What is a London dispersion force?
What is a London dispersion force?
Open in App
Solution
The correct option is A The weak intermolecular force that results from the motion of electrons that creates temporary dipoles in molecules.
London dispersion force is the strong intermolecular force that results from the motion of electrons that creates temporary dipoles in molecules.
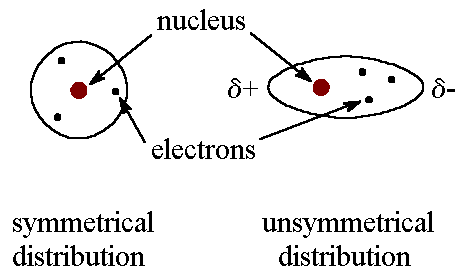
London dispersion force is the strong intermolecular force that results from the motion of electrons that creates temporary dipoles in molecules.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
