1
Question
What is the lone pair effect?
What is the lone pair effect?
Open in App
Solution
- Lone pair of electrons or non-bonded electrons are the electrons that do not take part in chemical bonding.
- When non-bonded electrons around an atom in a molecule are entirely shared by another atom or an ion it is called the lone pair effect.
- This leads to the formation of a coordinate bond.
- For example - etc. show this effect.
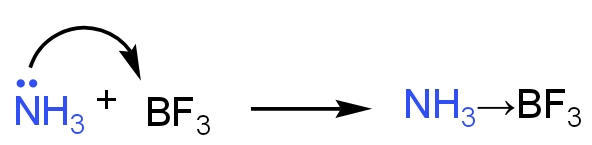
- Lone pair of electrons or non-bonded electrons are the electrons that do not take part in chemical bonding.
- When non-bonded electrons around an atom in a molecule are entirely shared by another atom or an ion it is called the lone pair effect.
- This leads to the formation of a coordinate bond.
- For example - etc. show this effect.
Suggest Corrections
4
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
