1
Question
Which substance below is resonance stabilized by delocalized pi electrons?
Which substance below is resonance stabilized by delocalized pi electrons?
Open in App
Solution
The correct option is D Benzene
(A) Benzene.
A conjugated six-membered ring is called benzene. In an aromatic system like benzene, each atom has a p orbital, the electrons of which are delocalized about the system. Benzene and other aromatic compounds exhibit a chemistry very different from ordinary, non-aromatic hydrocarbons. To distinguish between then, non-aromatic compounds are labelled aliphatic. Benzene and other aromatic compounds can have substituents. When benzene itself is a substituent, it is called a phenyl group. Benzene is typically drawn in such a way that the hybrid between the resonance structures is emphasized
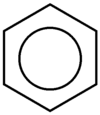
a conjugated cyclic system stabilized by resonance is described as aromatic. But not every conjugated cyclic system is aromatic since not all are stabilized by resonance, mainly due to differences in filling molecular orbitals with electrons. A system of rules was developed by chemist Erich Hückel to determine whether a system is aromatic:
(A) Benzene.
A conjugated six-membered ring is called benzene. In an aromatic system like benzene, each atom has a p orbital, the electrons of which are delocalized about the system. Benzene and other aromatic compounds exhibit a chemistry very different from ordinary, non-aromatic hydrocarbons. To distinguish between then, non-aromatic compounds are labelled aliphatic. Benzene and other aromatic compounds can have substituents. When benzene itself is a substituent, it is called a phenyl group. Benzene is typically drawn in such a way that the hybrid between the resonance structures is emphasized

a conjugated cyclic system stabilized by resonance is described as aromatic. But not every conjugated cyclic system is aromatic since not all are stabilized by resonance, mainly due to differences in filling molecular orbitals with electrons. A system of rules was developed by chemist Erich Hückel to determine whether a system is aromatic:
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
