1
Question
Electron sp3d2 hybridization by suitable example.
Open in App
Solution
In SF6, one electron each from 3s and 3p orbitals is promoted to 3d orbitals The six orbitals get hybridized to form six sp3d2 hybrid orbitals. Each of these sp3d2 hybrid orbitals overlaps with 2p orbital of fluorine to form S–F bond.
Thus, SF6 molecule has octahedral structure. The dotted electrons represent electrons from F–atoms.
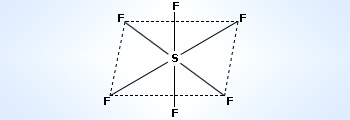
In SF6, one electron each from 3s and 3p orbitals is promoted to 3d orbitals The six orbitals get hybridized to form six sp3d2 hybrid orbitals. Each of these sp3d2 hybrid orbitals overlaps with 2p orbital of fluorine to form S–F bond.
Thus, SF6 molecule has octahedral structure. The dotted electrons represent electrons from F–atoms.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
