6
Question
KF crystallizes in the NaCl type structure. If the radius of K+ ions 132 pm and that of F− ion is 135 pm, what is the closest KF distance (in pm)?
KF crystallizes in the NaCl type structure. If the radius of K+ ions 132 pm and that of F− ion is 135 pm, what is the closest KF distance (in pm)?
Open in App
Solution
The correct option is D 378
NaCl has a cubic unit cell. It is best thought of as a face-centered cubic array of anions with an interpenetrating fcc cation lattice (or vice-versa). The cell looks the same whether you start with anions or cations on the corners. Each ion is 6-coordinate and has a local octahedral geometry.
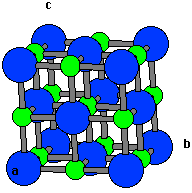
Length of unit cell = 2*(R(cation) + R(anion))
a = 534 pm
Closest K-K distance = a/√2 = 378 pm
NaCl has a cubic unit cell. It is best thought of as a face-centered cubic array of anions with an interpenetrating fcc cation lattice (or vice-versa). The cell looks the same whether you start with anions or cations on the corners. Each ion is 6-coordinate and has a local octahedral geometry.
 Length of unit cell = 2*(R(cation) + R(anion)) a = 534 pm Closest K-K distance = a/√2 = 378 pm |
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
