1
Question
The product(s) obtained via oxymercuation (HgSO4+H2SO4) of 1-butyne would be :
The product(s) obtained via oxymercuation (HgSO4+H2SO4) of 1-butyne would be :
Open in App
Solution
The correct option is A CH3−CH2−C||O−CH3
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol.
It
is electrophilic addition of water to double or triple bond, in
presence of mercury sulphate and sulphuric acid. Markownikoff product takes place. One can obtain both OH
product or RO product depending on whether one is using water or
alcohol.
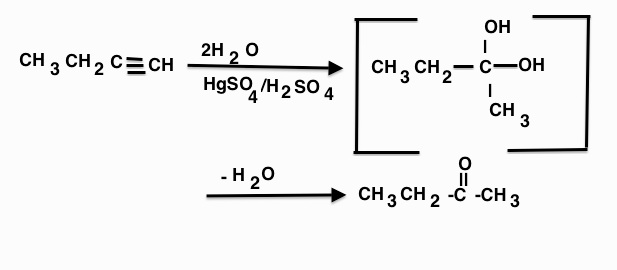
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol.
It is electrophilic addition of water to double or triple bond, in presence of mercury sulphate and sulphuric acid. Markownikoff product takes place. One can obtain both OH product or RO product depending on whether one is using water or alcohol.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
