Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. For an element, relative atomic mass is the average mass of the naturally occurring isotopes of that element relative to the mass of an atom of 12C. Which means one atom is given a relative atomic mass of exactly 12. Atomic mass is not reported with units. The formula for atomic mass is given below
Atomic mass = Mass of protons + Mass of neutrons + Mass of electrons
How to Calculate Atomic Mass?
1. By referring to the periodic table
In the periodic table digit of an atomic mass usually marked under the representation of an element. For example
Hydrogen (H) atomic mass -1
Lithium (Li) atomic mass -3
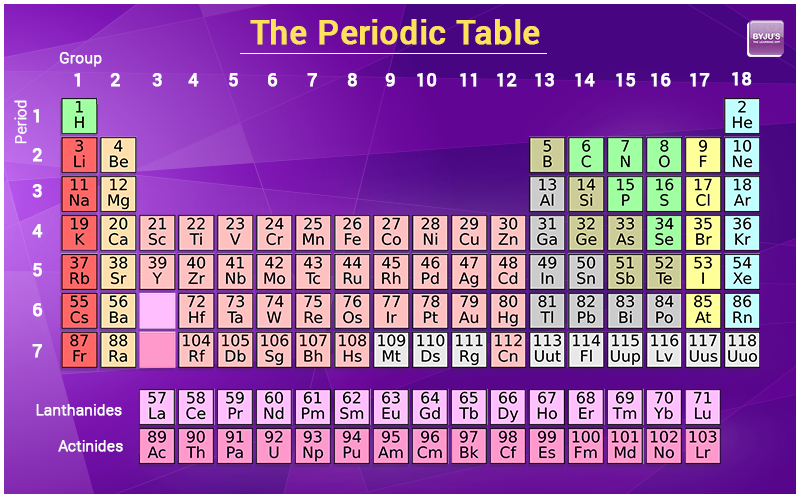
Periodic Table
2. Addition Of Mass Of Protons and Neutrons
In an element atomic mass of an atom can be calculated by adding the mass of protons and neutrons.
3. All Atoms of an Element – Weighted Average
3. Weight average of an element
Atomic Mass of Elements
The atomic mass of a few substances is tabulated below.
| Substance | Atomic mass |
| Calcium ion | 40.1 |
| Chloride Ion | 35.5 |
| Magnesium Ion | 24.3 |
| Potassium Ion | 39.1 |
Relative Atomic Mass
The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. By using this chemists work out the chemical formula. The relative atomic mass scale is used to compare the masses of different atoms.
First the hydrogen atom the lightest atom was originally assigned a relative atomic mass of 1 and the relative atomic mass of other atoms was compared with this.
| Element | Relative atomic mass |
| Carbon | 12 |
| Hydrogen | 1 |
| Oxygen | 16 |
| Magnesium | 24 |
| Nitrogen | 14 |
Atomic Mass Problems
Problem – 1: Find the element mass number whose atomic number is 18 and the neutron number is 20.
Solution:
| More topics in Atomic Mass Formula | |
| Avogadro’s Law Formula | Molecular Weight Formula |
| Weighted Average Formula | Gay Lussac Law Formula |
Learn more about the Atomic Mass Formula from the expert faculty at BYJU’S.
Comments