Calcium iodide is an inorganic compound that is made of calcium and iodine. It has a chemical formula CaI2. Calcium iodide can be formed using either calcium oxide, calcium carbonate or calcium hydroxide with hydrochloric acid. In this short piece of article, learn about the calcium iodide formula along with its chemical structure, uses and properties.
Calcium Iodide Properties
| Properties of Calcium Iodide | |
| Name | Calcium Iodide |
| Also Known as | Calcium (II) Iodide |
| Appearance | White Solid |
| Molecular Formula | CaI2 |
| Melting Point | 779 °C |
| Boiling Point | 1100 °C |
| Density | 3.956 g/cm3 |
| Molar Mass | 293.887 g/mol |
| Solubility in Water | Soluble |
Calcium Iodide Chemical Structure
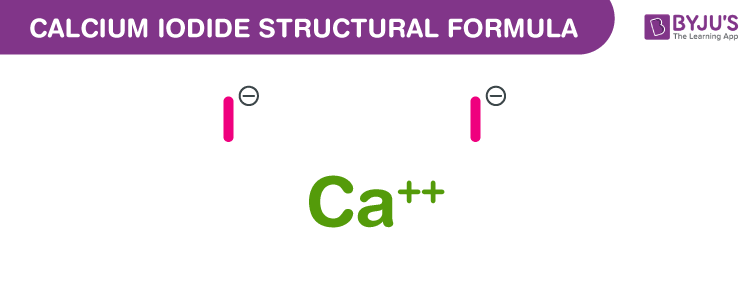
Calcium Iodide Uses
- Calcium iodide is used in medicines
- It is used in photography
- Calcium iodide is used as an iodine source in cat food.
To learn more about such chemistry topics register to BYJU’S now!

Comments