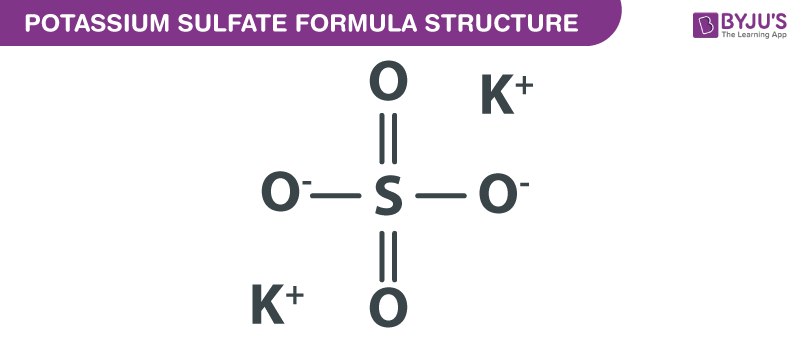
Potassium Sulfate formula, also known as Dipotassium sulfate formula or Sulphate Of Potash formula is explained in this article. This inorganic compound consists of one sulfur atom, four oxygen atoms, and two potassium atoms. From the four oxygen atoms attached to the sulfur atom, two of them are connected with double bonds and the other two with single bonds. The chemical or molecular formula of Potassium Sulfate is K2SO4.
It obtained as colourless or white crystals, or in powder form or as white granules. It has no smell but has a bitter, hard, saline taste. It can dissolve in water but cannot dissolve in ethanol. Naturally, it can be obtained from minerals abundant in the Stassfurt salt. It can be synthesized by reacting potassium chloride and sulfuric acid to produce sodium sulfate. In the exothermic reaction, an intermediate formation of potassium bisulfate occur. It is widely used in fertilizers to provide a source of potassium and sulfur.
Potassium Sulfate Formula Structure
Properties Of Potassium Sulfate Formula
| Chemical formula | K2SO4 |
| Molecular weight | 174.259 g/mol |
| Density | 2.66 g/cm3 |
| Boiling point | 1689 °C |
| Melting point | 1067 °C |
Inhaling K2SO4 leads to sore throat and coughing. When it comes in contact with eyes and skin it may cause redness and pain. Ingesting this compound causes nausea, vomiting, abdominal pain, diarrhoea, etc.
To learn more about Potassium Sulfate formula from the expert faculties at BYJU’S, register now!

Comments