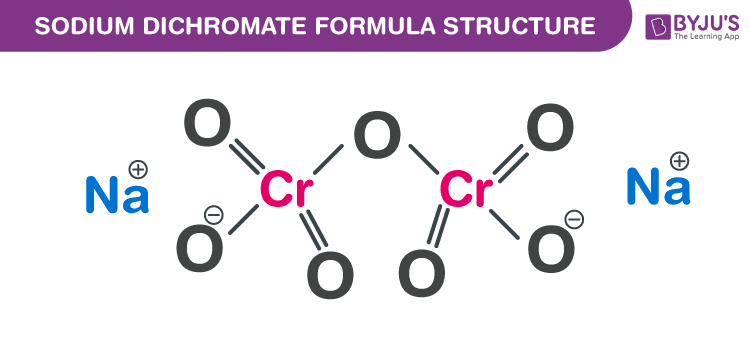
Sodium dichromate formula, also known as Disodium dichromate formula or Bichromate of soda formula is discussed in this article. It is an inorganic compound and emits toxic fumes of chromium when heated. It is extremely corrosive. The molecular or chemical formula of Sodium dichromate is Na2Cr2O7.
Disodium dichromate is a crystalline solid compound which is orange to red in colour. It is a strong oxidizing agent and is mostly used to obtain other chromium compounds. It mainly affects the respiratory system which causes shortness of breath, asthma. It also affects the gastrointestinal tract, the immune system. It is known as a human carcinogen which causes lung cancer.
Sodium dichromate Formula Structure
Properties Of Sodium dichromate Formula
| Chemical formula | Na2Cr2O7 |
| Molecular weight | 261.97 g/mol (anhydrous) |
| Density | 2.52 g/cm3 |
| Boiling point | 400 °C |
| Melting point | 356.7 °C |
Safety Measures
- It is a toxic compound.
- It is very corrosive and can be extremely irritating to eyes, mucous membranes, and skin.
Use Of Sodium Dichromate
- It is widely used in drilling muds.
- It is used as a wood preservative.
- Used in the production of organic chemicals.
- Used as a corrosion inhibitor also in metal treatments and in the manufacturing of dyes.
To learn more about Sodium dichromate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Bichromate of soda for free.

Comments