It is a white crystalline solid formed when ammonia reacts with acetic acid. It is widely used in the chemical analysis, in the pharmaceutical industry, the food sector in preserving foods, and in various other industries too. It is also used as a buffer in topical personal care and cosmetic products in manufacturing skin lotions, shampoos, conditioners and more.
Properties Of Ammonium Acetate Formula
| Chemical formula | NH4CH3CO2 |
| Molecular weight | 77.083 g/mol |
| Density | 1.17 g/cm3 (20 °C)
1.073 g/cm3 (25 °C) |
| Melting point | 113 °C (235 °F; 386 K) |
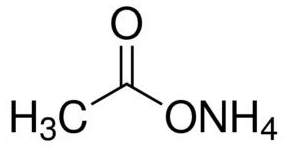
Ammonium Acetate Structural Formula
As Ammonium Acetate salt is constituted of a weak acid and a weak base and is often used with acetic acid to create a buffer solution. Ammonium acetate chemical component is volatile at low pressures because it has been used to replace cell buffers with non-volatile salts in preparing the chemical samples. The chemical formula is as given below.
For more scientific information, stay tuned with BYJU’S!!

Comments