Chlorous Acid formula, also known as Chlorige Saeure formula or Vicon formula is explained in this article. This inorganic is a weak acid and chlorine has an oxidation state of +3. This compound can be obtained by reacting barium or lead chlorite with dilute sulfuric acid. It consists of a one chlorine atom, one hydrogen atom, and two oxygen atoms. The chemical or molecular formula of Chlorous Acid is HClO2.
In its isolated form, it is a colourless liquid without a characteristic aroma. It is an unstable compound due to the dismutation reaction to obtain chloric and hypochlorous acid which is similar to that of bromine and iodine analogues. It is a strong oxidizing agent and incompatible with alkalis and reducing agents. It is widely used as a mouthwash for plaque reduction.
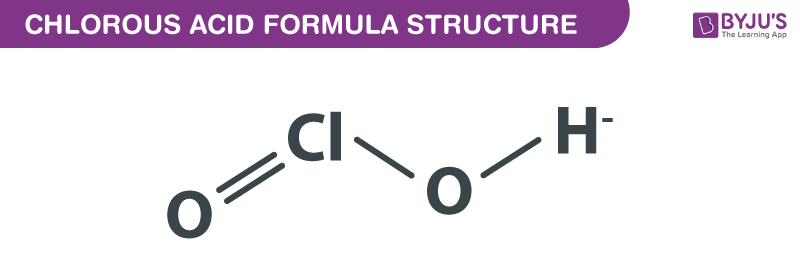
Chlorous Acid Formula Structure
Properties Of Chlorous Acid Formula
| Chemical formula | HClO2 |
| Molecular weight | 68.46 g/mol |
| Conjugate base | Chlorine |
| pKa | 1.96 |
| Boiling point | 502.07 °C |
When this compound comes in contact with skin and eyes or when ingested or inhaled it is very dangerous. If you have worn contact lenses and this acid goes into your eyes, remove them and flush your eyes with running water for 15 minutes by keeping your eyelids open. Do not use eye ointment. In case if the chemical comes in contact with clothing, quickly remove it and take a shower immediately.
To learn more about Chlorous Acid formula from the expert faculties at BYJU’S, register now!
Comments