Cobalt (II) Nitrate is a pale red powder colour crystalline compound. It is an odourless chemical that sinks and mixes with water. Cobalt (II) Nitrate is a nitrate salt in which the metal cobalt is seen in +2 oxidation state and the anion is nitrate. The other names of cobalt (II) are cobaltous nitrate, nitric acid, cobalt(2+) salt. Let us know more about the chemical composition of Cobalt (II) Nitrate.
| Chemical formula | Co(NO3)2 or CoN2O6 |
| Molecular weight | 182.941 g/mol (anhydrous)
291.03 g/mol (hexahydrate) |
| Density | 2.49 g/cm3 (anhydrous)
1.87 g/cm3 (hexahydrate) |
| Melting point | 100 °C (anhydrous)
55 °C (hexahydrate) |
| Boiling point | 100 to 105 °C (anhydrous)
74 °C (hexahydrate) |
Cobalt (II) Nitrate Structural Formula
Here is the typical structural representation of Cobalt II Nitrate.
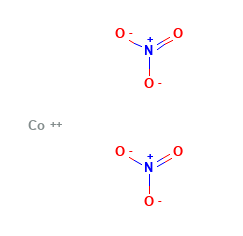
Anhydrous cobalt(II) nitrate utilizes a three-dimensional polymeric network structure, where each cobalt atom is octahedrally connected to 6 oxygen atoms.
Use Of Cobalt (II) Nitrate
- Used in the synthesis of ink and dyes.
- It is used to reduce the high purity cobalt.
For more understanding of the concept refer to BYJU’S!!

Comments