Iron III chloride, which is otherwise known as ferric chloride is a compound made of iron and chloride with the formula FeCl3. It rarely occurs in its natural form which is the mineral molysite. Although, it can be industrially manufactured by combining iron and chlorine as follows:
\(\begin{array}{l}2Fe+3Cl_2\rightarrow 2FeCl_3\end{array} \)
In this article, learn more about the Iron III Chloride formula, its chemical structure, properties and uses.
Iron III Chloride Properties
| Properties of Iron III Chlorine | |
| Name | Iron III Chlorine |
| Other Names | Molysite, Ferric Chloride, Flores martis |
| Chemical Formula | FeCl3 |
| Melting Point | 307.6 °C |
| Boiling Point | 316 °C |
| Molar Mass | 162.2 g/mol |
| Density | 2.9 g/cm³ |
| Solubility in Water | Soluble |
Iron III Chloride Structure
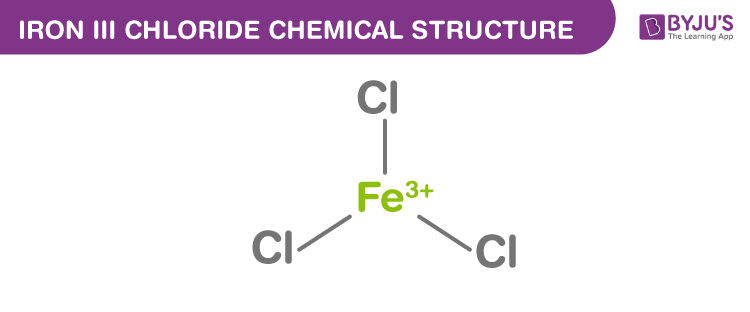
Iron III Chloride Uses
- Used in drinking water production and sewage water treatment
- Used as a catalyst for the reaction between chlorine and ethylene
- Used in energy storage systems
- Used as a drying reagent in certain reactions
- In laboratory FeCl3 used as lewis acid (catalysing agent) for Friedel-craft reaction, chlorination of aromatic compounds.
To learn more about such chemistry topics register to BYJU’S now!

Comments