Lead Iodide formula, also known as Lead (II) Iodide formula or Plumbous Iodide formula is discussed in this article. It is a highly toxic salt and has very poor stability. The molecular or chemical formula of Lead Iodide is PbI2. Plumbous Iodide is a crystalline solid bright yellow in colour at room temperature. The colour changes to orange and further to red on heating it. It has no smell and is denser than water. It does not dissolve in water. It is widely used photography, printing, manufacturing of solar cells, etc. It can be synthesized by a reaction called a double displacement reaction. In this reaction potassium iodide and lead (II) nitrate are made to react in water solution.
Lead Iodide Formula Structure
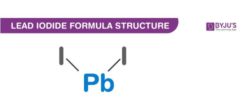
Properties Of Lead Iodide Formula
| Chemical formula | PbI2 |
| Molecular weight | 461.01 g/mol |
| Density | 6.16 g/cm3 |
| Boiling point | 953 °C |
| Melting point | 402 °C |
Safety Measures
- It is hazardous and a threat to the environment. Therefore quick measures need to be taken to stop spreading it to the environment.
- Plumbous Iodide is very toxic to human health and carcinogen to animals.
To learn more about Lead Iodide formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Plumbous Iodide for free.

Comments