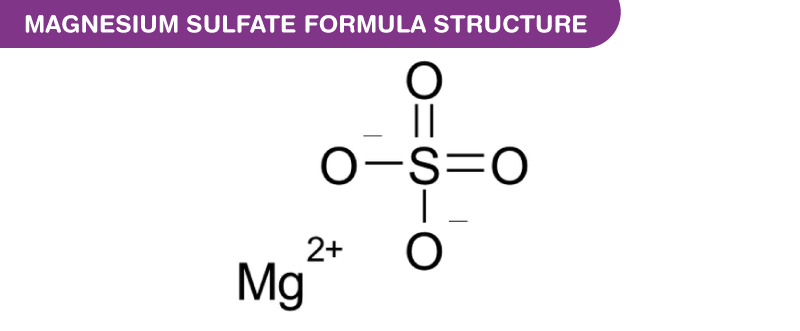
Magnesium Sulfate formula, also known as Epsom Salt formula or Bath Salt formula is explained in this article. This inorganic salt consists of a salt composed of Mg2+ (bivalent magnesium cation) and SO42- (sulfate anion). The sulfur atom in the centre is connected to four oxygen atoms. These oxygen atoms are attached through two single bonds and two double bonds. The chemical or molecular formula of Magnesium Sulfate is MgSO4.
It occurs as a colourless to white crystalline solid or opaque powder. It has no odour and has a bitter taste or saline taste. It dissolves in water, glycerin, ether, alcohols, and glycerol. It does not dissolve in acetone. It can be prepared commercially by the chemical reaction of sulfuric acid with either magnesium hydroxide or magnesium oxide, and magnesium carbonate.
Magnesium Sulfate Formula Structure
Properties Of Magnesium Sulfate Formula
| Chemical formula of Magnesium Sulfate | MgSO4 |
| Molecular weight of Magnesium Sulfate | 120.366 g/mol (anhydrous) |
| Density of Magnesium Sulfate | 2.66 g/cm3 (anhydrous) |
| Boiling point | decomposes |
| Melting point of Magnesium Sulfate | 1,124 °C |
This compound is not toxic but when heated to decompose it gives out fumes of sulfur oxides which is toxic as well as corrosive.
To learn more about Magnesium Sulfate formula from the expert faculties at BYJU’S, register now!

Comments