Sulfur Trioxide formula, also known as Sulfan formula or Oxosulfane dioxide formula is explained in this article. It is corrosive to metals and violently reacts with water to form sulfuric acid. The chemical or molecular formula of Sulfur Trioxide is SO3.
Sulfan is obtained as a colourless to white crystalline solid. It dissolves in water and reacts to give sulfuric acid. Its odour depends on its form. In its vapour form, it has a pungent smell like sulfur dioxide whereas mist has no smell. On an industrial scale, it is prepared as a precursor to sulfuric acid. In the laboratory, Sulfan can be prepared by two-stage pyrolysis of NaHSO4. Sodium pyrosulfate is an intermediate product. It is widely used in sulfonation reactions as an essential reagent.
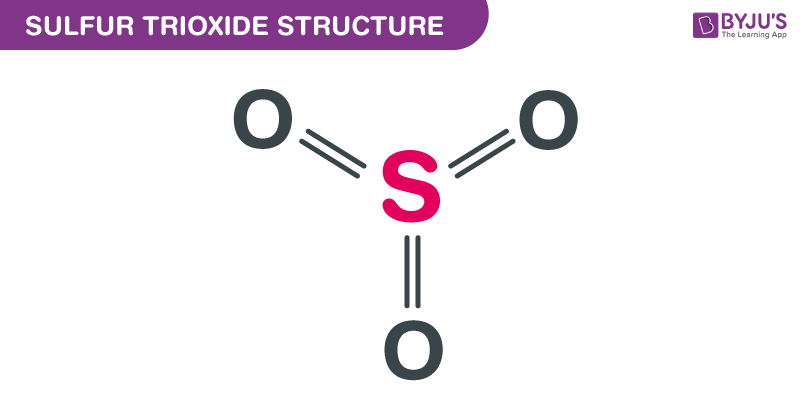
Sulfur Trioxide Formula Structure
Properties Of Sulfur Trioxide
| Chemical formula | SO3 |
| Molecular weight | 80.066 g/mol |
| Density | 1.92 g/cm3 |
| Boiling point | 45 °C |
| Melting point | 16.9 °C |
Oxosulfane dioxide is highly hygroscopic. The heat of hydration is sufficient for the mixtures of Sulfur Trioxide, wood or cotton to ignite. It is hazardous as it can cause severe eye and skin burns. Ingesting this compound results in severe burns of mouth, stomach, and oesophagus.
To learn more about Sulfur Trioxide formula from the expert faculties at BYJU’S, register now!

Comments