West Bengal Madhyamik Board 10th Standard Physical Science Exam Question Paper 2015- Free Download
The West Bengal Board Class 10 Physical Science 2015 Question Paper is used to help the students to study for the board exams of Class 10. The Madhyamik previous year paper also lays the foundation for the question types that are likely to be asked in the Physical Sciences exams. Practising the West Bengal Board Class 10 Physical Science 2015 Question Paper help students to face the exams more confidently.
Students can download this question paper by clicking the relevant link here and then answer the unsolved question paper for practice. Students can also click on the interactive PDF link and access the printable format for the question paper. The questions and answers are also available on the webpage.
Madhyamik Students, meanwhile, are advised to get familiar with the key topics or concepts that are covered in the West Bengal Board Class 10 Physical Science textbook. The below given question paper is considered as a perfect resource for the students as it helps them to get an overview of the question paper format of the WBBSE Class 10 Physical Science exam. Madhyamik Board Students are urged to practise this previous year paper of West Bengal Board Class 10 Physical Science and revise the subject competently. Referring to the question paper will help the students to gauge their performance and preparation level for the board exams. The students can also solve previous year papers on a regular basis to get an idea on how to answer the questions most proficiently.
Download West Bengal Madhyamik Board 2015 Physical Science Question Paper
West Bengal Madhyamik Board Class 10 Physical Science 2019 Question
Group-A
- Give very short answers to any ten questions: [1×10=10]
1.1 What is the total number of charged particles in 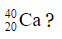
1.2 Express 327 °C in Kelvin.
1.3 What is the number of molecules in 2.24 litre SO2 at STP.
1.4 What is the relation between 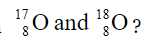
1.5 Specific heat of which liquid is the highest?
1.6 In Flemings left hand rule the direction of which is indicated by the forefinger?
1.7 What type of lens is used to convert a parallel beam of light into a converging beam?
1.8 Write the name of the radioactive ray which has no charge.
1.9 To which group of Mendeleev’s periodic table do the alkali metals belong?
1.10 Which acid can be detected by ring test?
1.11 Which type of chemical bond is present in hydrogen chloride molecule?
1.12 Write the name of an organic greenhouse gas.
1.13 How many hydrogen atoms are present in an alkaline containing three carbon atoms?
Group-B
2.1 What is the nucleus of an atom?
2.2.1 Write the name of two particles present in the nucleus of an atom (except hydrogen atom)
2.2.2 Why do chemical properties of Isotopes remain the same?
2.3 Why the electronic configuration of an atom having atomic number 12 cannot be K(2), L(9), M(1)? What will be the electronic configuration of the atom?
3.1 State Boyle’s law and express it in Mathematical form.
3.2 Express the combined Boyle’s law and Charle’s law in the form of an equation. What do the symbols used in this equation indicate?
3.3 At 90 cm Hg pressure the volume of a fixed mass of gas is 500 cm3. What will be the volume of the gas at 60 cm Hg pressure at the same temperature?
4.1 State Avogadro’s law and mention two of its deductions.
4.2 Define atomic mass of an element m C-12 scale.
4.3 How many grams of sulphur trioxide will contain the same number of molecules as present in 6.4 gram of sulphur dioxide? (O =16, S =32)
5.1 A bi positive ion is produced from an atom. Which particle will differ in number between the atom and the ion? In which, the ion or the atom, this particle will be present in greater number and by how many?
5.2.1 Whose unit is mole?
5.2.2 Write with an example what is meant by gram molecular mass.
5.3 How many grams of (i) magnesium chloride (ii) hydrogen will be produced when 36gms of magnesium reacts with excess dilute hydrochloric acid?
(H=1, Mg=24, Cl=35.5)
Group-C
6.1 On what factors do the heat taken or given out by a body depend and how?
6.2 Write the fundamental principle of calorimetry.
6.3.1 Write the SI unit of heat.
6.3.2 How much heat will be required to raise the temperature of 250g of water from 10 °C to 100 °C?
7.1.1 What is called a Convex lens?
7.1.2 What is meant by the focal length of a Convex lens?
7.2 Mention two differences between real and virtual image.
7.3 What is called dispersion of light?
8.1 What is linear magnification of an image formed by a lens?
8.2 State Ohm’s law. How is the definition of resistance obtained from this law?
8.3 In which combination are the three conductors of resistances 2 ohm, 3 ohm and 4 ohm to be connected so that the same current flows through each of them? Find the equivalent resistance of the combination.
9.1 On what factors do the heat produced due to flow of electric current in a conductor depend and how?
9.2 Name two instruments we use in daily life which utilise the heating effect of current.
9.3.1 Which one is the earth pin of a three pin top?
9.3.2 Mention two characteristics of a fuse wire.
10.1 An electric current is flowing through a wire. If a magnetic needle is placed under the wire parallel to it, the needle is deflected. Write the rule regarding the direction of deflection of the magnetic needle.
10.2 What effect is observed on the rotation of a Barlow’s wheel when
i) Strength of the magnet is increased
ii) Direction of electric current is reversed
10.3.1 What is an electromagnet?
10.3.2 The electromotive force of a cell is E volt. How much work is to be done when 1 coulomb of charge is taken from positive pole to negative pole of the cell?
11.1 What is called thermionic emission?
11.2 Mention one property and one use of X-Ray.
11.3.1 What is natural radioactivity?
11.3.2 Mention two characteristics of nuclear fusion.
Group- D
12.1 Let the symbols of two elements be A and B and their atomic numbers are 7 and 20, respectively.
i) Indicate the position of A and B in Mendeleev’s periodic table
ii) If two atoms of element A combine to form a molecule, what will be the nature of the bond formed in the molecule? Show the electron dot structure of the molecule.
12.2 Show according to the electronic theory of oxidation reduction that oxidation and reduction occur simultaneously in the following reaction:
2Fe3+ + Sn2+ = 2Fe2+ + Sn4+
12.3 Write the reactions which occur at cathode and anode when aqueous copper sulphate solution acidified with sulphuric acid is electrolysed using copper electrodes.
13.1.1 Mention the following for the laboratory preparation of hydrogen chloride:
(i) chemicals required
(ii) balanced chemical equation
13.1.2 Why cannot hydrogen chloride gas be collected by downward displacement of water? How is hydrogen chloride gas collected?
13.2 What is fuming nitric acid?
13.3 Give one example of oxidation reaction of concentrated sulphuric acid with balanced chemical equation.
14.1 Give an example of one ore each of iron and aluminium.
14.2 Write structural formulas of two organic compounds having the same molecular formula but different properties.
14.3 Match the right column with the left column:
| Left Column | Right Column |
| (a) Organic insect repellent insoluble in water | (i) CH3COOH |
| (b) Main constituent of methylated spirit and rectified spirit | (ii)Naphthalene |
| (c) Present in both brass and bronze | (iii) Cr |
| (d) Present in vinegar | (iv) CH3CH20H |
| (e) Present in stainless steel | (v) Zn |
| (f) Used in the detection of the presence of traces amount of water in a solution | (vi) slaked lime |
| (g) Used in the preparation of bleaching powder | (vii) Anhydrous CuSO4 |
| (h) Present in brass and not in bronze | (viii) CuSO4 |
15. 1 Arrange the following atoms in the increasing order of electronegativity:
Cl, Br, I, F
15.2 White fumes are formed when a glass rod dipped in ammonia is held over the open mouth of a bottle containing concentrated acid. What is the acid? Why are white fumes formed? Write the balanced equation of the reaction when an aqueous solution of AgNO3 is added to the dilute solution of acid.
15.3 What will be the visual change when excess acetylene gas is passed in liquid bromine? What is the chemical reaction that occurs? Write the balanced chemical equation of the reaction.
16.1 Mention the monomer and one use of Teflon.
16.2 Mention two dangers of random use of polythene bags.
16.3.1 How is H2SO4 prepared from SO3 in contact process?
16.3.2 Which air polluting gas is liberated when Cu is heated with concentrated sulphuric acid? Mention another gas which is responsible for acid rain.
17.1 What is meant by electrolyte? Choose electrolyte and non-electrolyte from the following:
aqueous solution of sugar, fused sodium chloride, liquid HCl, mercury
17.2 Write the structural formula of a carboxylic acid containing three carbon atoms. Write the formula of the functional group present in it.
17.3 Write balanced chemical equations of the following two reactions:
i) Magnesium is added to dilute sulphuric acid
ii) Methane gas is burnt in oxygen




Comments