Glucose is a natural form of sugar formed by plants during the process of photosynthesis. Glucose is also found in variable levels in the blood sugar of humans and animals. Glucose is a monosaccharide sugar which is white in color and is soluble in water.
Table of Content
Chemical Formula Of Glucose
| Chemical formula | C6H12O6 |
| Molecular weight | 180.156 g/mol |
| Chemical names | dextrose, blood sugar, corn sugar and grape sugar |
| Molar mass | 180.156 g·mol-1 |
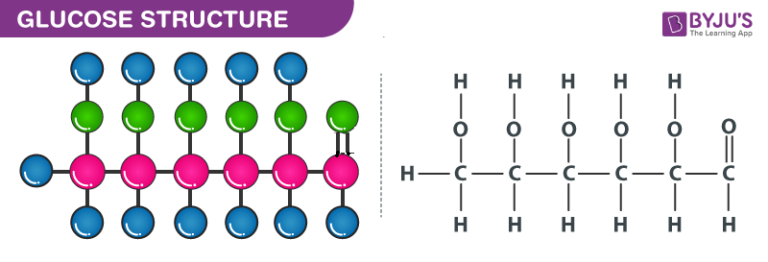
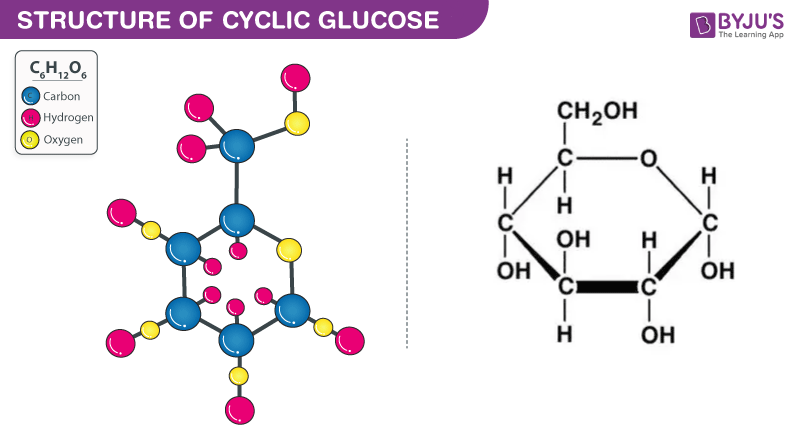
Structural Formula of Glucose
Structural formula of Glucose is as shown below. Glucose contains 6 carbon atoms and belongs to aldehyde group. Glucose may be found in either linear form or cyclic form. Glucose is the basic energy contributor for the human body.
- For more information about the structure of glucose you may visit: structure of glucose
Characters suggest the structure of Glucose
The open chain structure of glucose is CH2OH(CHOH)4CHO. The following characteristics suggest the structure of glucose.
Reaction due to alcoholic (OH) groups:
There are four secondary and one primary alcoholic groups present in the molecule of glucose. The reactions are given below.
Upon acetylation with acetyl chloride or acetic anhydride , glucose forms penta acetyl derivatives. This shows that the five -OH group is attached to five different carbon atoms.
CHO(CHOH)4CH2OH + 5(CH3CO)2O → CHO(CHOCOCH3)4CH2OCOCH3 + 5CH3COOH
Reaction:
Glucose is reduced with HI and red phosphorus upon prolonged heating produces n-hexane. This suggests that all the six carbon atoms in the glucose are linked to form a straight chain.
CHO(CHOH)4CH2OH ⟶ CH3CH2CH2CH2CH2CH3
Glucose n- hexane
Oxidation:
With a mild oxidizing agent like bromine water, glucose is oxidised to gluconic acid which is a monocarboxylic acid with 6 carbon atoms. This shows that the carbonyl group present in glucose is an aldehydic group.
CHO(CHOH)4CH2OH ⟶ CH2OH(CHOH)4COOH
Glucose Gluconic acid
When oxidised with a strong oxidizing agent like concentrated HNO3 , glucose is oxidised to a dicarboxylic acid saccharic acid. The formation of di carboxylic acid shows the presence of a primary alcoholic group. We may now conclude that out of these one is primary alcoholic group while other four are secondary alcoholic groups
CHO(CHOH)4CH2OH ⟶ COOH(CHOH)4COOH
Glucose Saccharic acid
Chemical properties of Glucose:
Action with Tollen’s reagent:
Tollen’s reagent is an ammoniacal silver nitrate solution reacting with a glucose solution, and a shining mirror gets deposited at the bottom of the tube.
CHO(CHOH)4CH2OH + 2Ag(NH3)2OH ⟶ COOH(CHOH)4CH2OH + 2Ag + 4NH3 + H2O
Action with Fehling’s solution:
When Fehling solutions heated with glucose solution give red precipitate of Cu2O.
CHO(CHOH)4CH2OH + 2Cu2+ + 4OH– ⟶ COOH(CHOH)4CH2OH + Cu2O + H2O
- For the preparation and FAQs of glucose you may visit: Glucose-C6H12O6
Refer BYJU’S for informative Videos and information!!
Comments