Aluminum Iodide is the simple compound formed when Aluminum reacts with iodine and is a white or yellow colour in nature. Aluminum Iodide is a strong Lewis base which means it contains an empty orbital capable of accepting an electron pair from a Lewis base to form a Lewis product. It used to break the chemical bond between compounds like C-O and N-O. Aluminum Iodide is used as an additive for electrolytes in lithium-ion batteries.
Properties Of Aluminium Iodide
| Chemical formula | AlI3 |
| Molecular weight | 407.695 g/mol (anhydrous)
515.786 g/mol (hexahydrate) |
| Density | 3.98 g/cm3 (anhydrous)
2.63 g/cm3 (hexahydrate) |
| solubility | Soluble in water |
| Boiling Point | 382 °C (anhydrous) |
| Crystalline structure | Monoclinic |
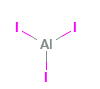
Aluminum Iodide Structural Formula
The structural depiction of Aluminum Iodide is as shown in the diagram below.
Stay tuned with BYJU’S to more scientific figures and facts!!

Comments