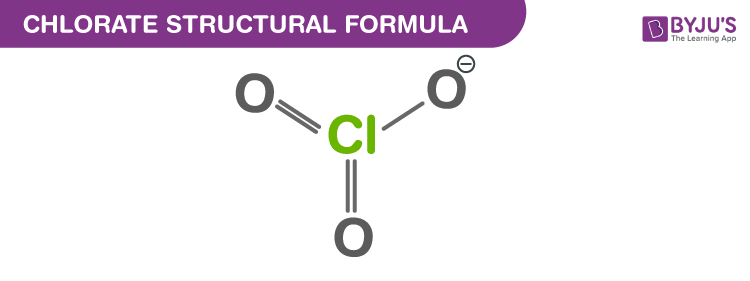
Chlorate or the chlorate ion has a molecular formula ClO3–. Chlorates are the salts of chloric acid. Chlorate when accompanied by a Roman numeral in parentheses, e.g. chlorate(VII), refers to a particular oxyanion of chlorine. Metal chlorates can be prepared by adding chlorine to metal hydroxides such as the KOH. In this short piece of article, let us discuss the chlorate formula, its properties and chemical structure along with the uses of chlorate.
Chlorate Structural Formula
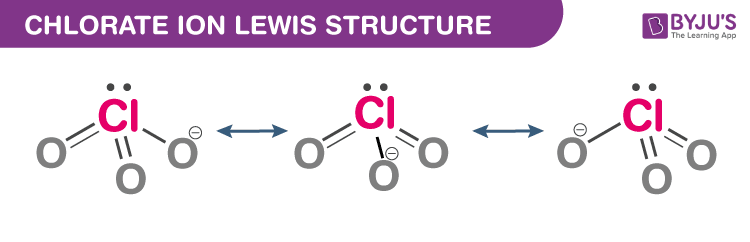
Chlorate Ion Lewis Structure
Just one Lewis structure cannot satisfactorily represent the chlorate ion since all the Cl–O bonds are of the same length and the chlorine atom is hypervalent. Rather, it is often considered as a hybrid of multiple resonance structures as follows:
Examples of Chlorates
Examples of chlorates include:
- Sodium Chlorate (NaClO3)
- Potassium Chlorate (KClO3)
- Magnesium Chlorate (Mg(ClO3)2)
If a Roman numeral in brackets follows the word “chlorate”, this indicates the oxyanion contains chlorine in the indicated oxidation state, namely:
As discussed, the Roman numeral that follows the word chlorate indicates the oxyanion that contains chlorine in the indicated oxidation state, namely
| Common Name | Stock name | Oxidation state | Formula |
| Hypochlorite | Chlorate(I) | +1 | ClO– |
| Chlorite | Chlorate(III) | +3 | ClO2– |
| Perchlorate | Chlorate(VII) | +7 | ClO4– |
To learn more about such chemistry topics register to BYJU’S now!

Comments