The glutaric acid formula, also known as Pentanedioic acid formula or n-Pyrotartaric acid formula is discussed in this article. It is an alpha,omega-dicarboxylic acid which has simple 5 carbon linear dicarboxylic acid (HO2C−R−CO2H). The molecular or chemical formula of Glutaric acid is C5H8O4.
When pentanedioic acid is present in a high amount it acts as a metabotoxin and as an acidogen. It can be synthesized by the following process
- The ring-opening of butyrolactone (C4H6O2) with potassium cyanide (KCN) to produce potassium carboxylate-nitrile. It is hydrolyzed further to diacid. Oxidizing dihydropyran will produce glutaric acid.
- It can also be synthesized by treating 1,3-dibromopropane with potassium or sodium cyanide to produce dinitrile. Further, it is hydrolysed to obtain glutaric acid.
Glutaric acid Formula Structure
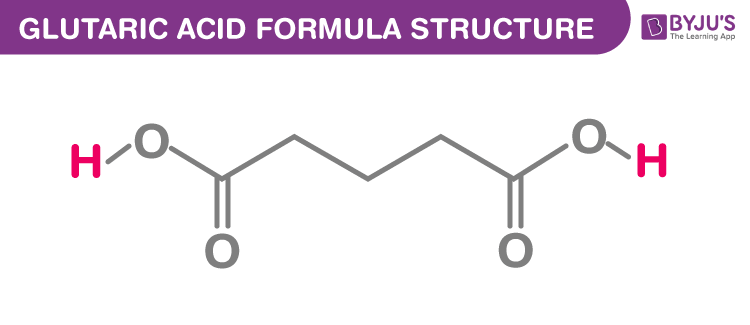
Properties Of Glutaric acid Formula
| Chemical formula | C5H8O4 |
| Molecular weight | 132.12 g/mol |
| Melting point | 95 to 98 °C |
| Boiling point | 200 °C |
n-Pyrotartaric acid is naturally produced by the body during the process of metabolism of certain amino acids, which include tryptophan and including lysine. Any defect in the pathway can cause disorder such as glutaric aciduria which results in severe encephalopathy.
Use Of Glutaric Acid
- Hydrogenation of glutaric acid and its derivatives produces a placticizers.
- Used to produce many polymers such as polyesters, polyamides.
To learn more about Glutaric acid formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Pentanedioic acid for free.
Why is it called ” glutaric” acid??