Hydrazine formula, also known as Nitrogen hydride formula or Diazane formula is discussed in this article. Nitrogen hydride is a highly reactive base as well as a good reducing agent which is used in many medical and industrial applications. The molecular or chemical formula of Hydrazine is N2H4. In its anhydrous form, it is a colourless compound. It is a fuming oily liquid and smells like ammonia. There are chances of exploding during distillation if any traces of air are found. It is mainly used in fuel cells and as a rocket propellant.
Hydrazine Formula Structure
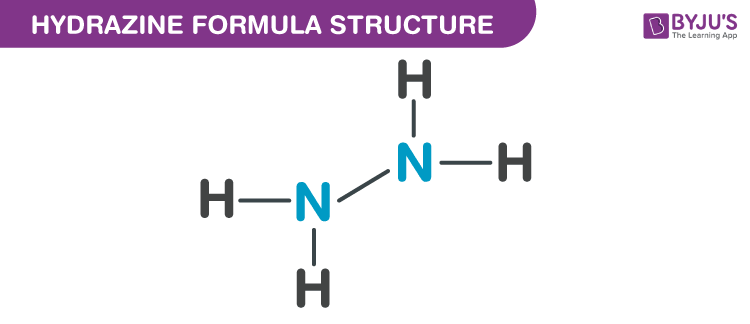
Properties Of Hydrazine Formula
| Chemical formula | N2H4 |
| Molecular weight | 32.0452 g/mol |
| Density | 1.021 g/cm3 |
| Boiling point | 114 °C |
| Melting point | 2 °C |
In its natural form, it is obtained by some microorganisms viz bacteria, fungi, and yeast. Commercially it can be prepared as by a process called “Raschig”. In this process solution of sodium hypochlorite is treated with ammonia in excess quantity to produce a chloramine intermediate. The final product obtained is hydrazine and hydrochloric acid.
To learn more about Hydrazine formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Nitrogen hydride for free.

Comments