Hydrogen sulfate, also known as bisulfate is a salt of sulfuric acid and is an ion with a chemical formula HSO4–. Chemical compounds with this ion are known as hydrogen sulfates or bisulfates. They are acidic in nature and can be used as a weaker form of acid than sulfuric acid. The formula of hydrogen sulfate indicates that it contains one atom of hydrogen and sulfur each with four atoms of oxygen. The ion carries a charge of –1. In this article learn the hydrogen sulfate formula, its chemical structure, properties and uses.
Hydrogen Sulfate Properties
| Properties of Hydrogen Sulfate | |
| Name | Hydrogen Sulfate |
| Also Known as | Bisulfate |
| Molecular Formula | HSO4– |
| Melting Point | 58.5 °C |
| Density | 2.345 g/cm3 |
| Molar Mass | 97.0715 g/mol |
| Solubility in Water | Soluble |
Hydrogen Sulfate Chemical Structure
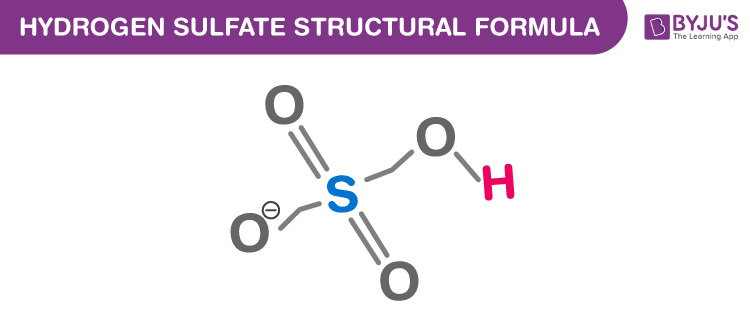
To learn more about such chemistry topics register to BYJU’S now!

Comments