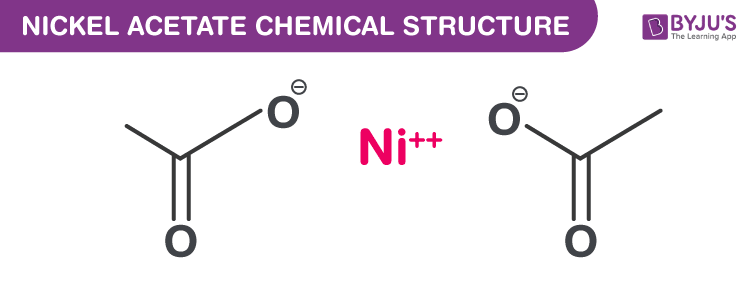
Nickel acetate is a moderately water-soluble chemical compound with a chemical formula Ni(CH3CO2)2. This compound can be obtained by treating nickel carbonate with acetic acid. The nickel acetate crystals have been seen to adopt an octahedral structure with nickel at the centre coordinated by four water molecule and two acetate ligands. In this short piece of article, learn more about the nickel acetate formula, its chemical structure along with its properties and uses.
Properties of Nickel Acetate
| Name | Nickel Acetate |
| Appearance | Green Solid |
| Chemical Formula | C4H6NiO4 |
| Other Name | Nickelous acetate, Nickel(II) acetate, Nickel diacetate |
| Molecular Weight | 248.841 g/mol |
| Density | 1.78 g/cm3 |
| Melting Point | Decomposes on heating |
| Solubility In Water | Soluble |
Nickel Acetate Chemical Structure
Nickel Acetate Uses
- Used as a mordant in the textile industry
- Used in anodizing applications
Safety Measurements
Nickel salts irritate the skin due to its carcinogenic nature.
Stay tuned to BYJU’S to learn more formulas of various other chemical compounds.

Comments