Nickel Sulfate, also known as Nickel (II) Sulfate, is an inorganic compound with a chemical formula NiSO4. It is usually obtained as a byproduct of copper refining. Nickel sulfate naturally occurs as a rare mineral known as retgersite. In this short piece of article, we shall discuss the nickel sulfate formula, its chemical structure along with a few of its properties and uses.
Nickel Sulfate Properties
| Properties of Nickel Sulfate | |
| Name | Nickel Sulfate |
| Other Names | Nickelous Sulfate, Nickel (II) Sulfate |
| Appearance | Yellow solid |
| Chemical Formula | NiSO4 |
| Melting Point | > 100 °C |
| Boiling Point | 840 °C |
| Density | 4.01 g/cm3 |
| Molar Mass | 154.75 g/mol |
| Solubility in Water | Soluble |
Nickel Sulfate Structure
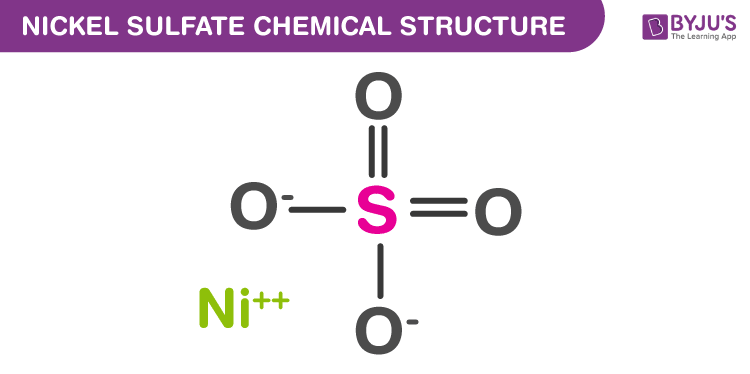
Nickel Sulfate Uses
- Used in laboratories
- Used as a calibrant for magnetic susceptibility measurements
- Used to make other nickel compounds
- Used in the electroplating of nickel on other metals.
To learn more about such chemistry topics register to BYJU’S now!
Comments