Potassium fluoride is a chemical compound that is the primary source of fluoride ion after hydrogen fluoride. KF is the molecular formula of potassium fluoride. It is a colourless or white crystalline compound with no odour. In this short piece of article, we will be discussing the potassium fluoride formula, its chemical structure, properties and uses.
Potassium Flouride Properties
| Properties of Potassium Fluoride | |
| Name | Potassium Fluoride |
| Also Known as | Potassium Monofluoride |
| Appearance | White powder or crystals |
| Chemical Formula | KF |
| Melting Point | 858 °C (anhydrous)
41 °C (dihydrate) 19.3 °C (trihydrate) |
| Boiling Point | 1,505 °C |
| Density | 2.48 g/cm³ |
| Molar Mass | 58.0967 g/mol (anhydrous)
94.1273 g/mol (dihydrate) |
| Solubility in Water | Soluble |
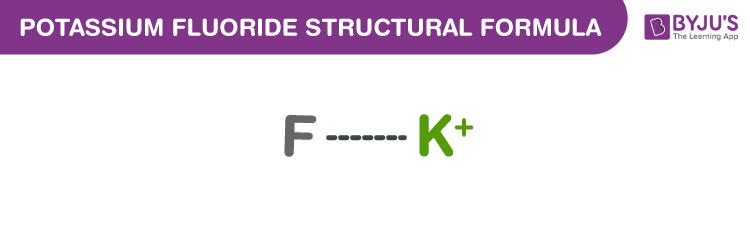
Potassium Fluoride Chemical Structure
Potassium Fluoride Uses
- Used as a fluoridating agent in the precipitation of organic chemicals
- Used as a flux in the field of metallurgy
- Used in the manufacture of optical glasses
- Used to make disinfectants, pesticides and insecticides
Safety Measurements
- KF is poisonous in nature.
- Inhalation of KF is very harmful.
- KF is very corrosive in nature, it may cause severe burn when it been in contact with skin.
To learn more about such chemistry topics register to BYJU’S now!

Comments