The Punjab School Education Board offers a wide range of topics in Chemistry for Class 12. Whether you want to pursue a career in engineering or other fields related to the subject, such as Pharmaceuticals, you will have the perfect foundation to pursue higher studies. We have included Punjab Board Class 12 Chemistry Syllabus, so you can start your preparations in earnest.
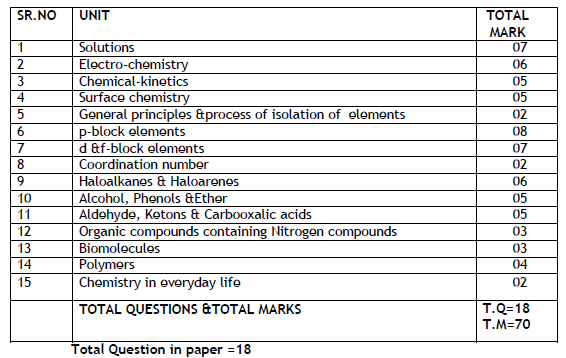
To get an idea about the reduced PSEB Class 12 Chemistry syllabus 2021-22 and to get details of the deleted portions of the syllabus for the current academic year, students can click on these links provided and access the respective PDFs. The Unit wise marks distribution for board exam papers of previous year are also shown as screenshot below, in this article.
Download PSEB Class 12 Chemistry Syllabus PDF 2021-22
From traditional science topics such as Electrochemistry to more modern topics such as Polymers, the exhaustive list of Chemistry syllabus is all that you need to prepare for the Chemistry exams of Punjab Board Class 12 .
Unitwise Distribution Of Marks For Board Exam Papers 2020-2021 Format
Punjab board has also included a right mix of practicals in the syllabus, so the students can also gain application perspective. See, here the list of Practical syllabus:
PSEB CLASS 12 Chemistry Practical Syllabus
| A. Surface Chemistry
a. Preparation of one lyophilic and one lyophobic sol. Lyophilic sol -starch, egg albumin and gum. Lyophobic sol – aluminum hydroxide, ferric hydroxide, arsenious sulphide. b. Study of the role of emulsifying in stabilizing the emulsions of different oils. |
| B. Chemical Kinetics
a. Effect of concentration and temperature on the rate of reaction between sodium thiosulphate and hydrochloric acid. b. Study of reaction rates of any one of the following:- i. Reaction of iodide ion with hydrogen peroxide at room temperature using different concentration of iodide ions. ii. Reaction between potassium iodate, KI03, and sodium sulphite : (Na2,SO3) using starch solution as indicator( clock reaction) |
| C. Thermochemistry: Any one of the following experiments
a. Enthalphy of dissolution of copper sulphate or potassium nitrate. b. Enthalphy of neutralization of strong acid (HCl) and strong base (NaOH) c. Determination of enthalpy change during interaction (Hydrogen bond formation) between acetone and chloroform. |
| D. Electrochemistry: Variation of cell potential in Zn/Zn+2 II Cu+2/Cu will change in concentration of electrolytes (CuSO4 or ZnSO4 at room temperature. |
| E. Chromatography
a. Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values. b. Separation of constituents present in an inorganic mixture containing two cations only (constituents having wide difference in Rf, values to be provided). |
| F. Determination of concentration/molarity of KMnO4 solution by titrating it against a standard Solution of:
a. Oxalic acid. b. Ferrous ammonium sulphate. (Students will be required to prepare standard solutions by weighing themselves). |
| G. Preparation of Inorganic Compounds
a. Preparation of double salt of ferrous ammonium sulphate or potash alum. b. Preparation of potassium ferric oxalate. |
| H. Preparation of Organic Compounds: Preparation of any two of the following compounds
a. Acetanilide b. Di-benzal acetone c. p-Nitroacetanilide, d. Aniline yellow òr 2-Naphthol aniline dye. e. Lodoform |
| I. Test for the functional groups present in organic compounds: Unsaturation, alcoholic, pheholic, aldehydic, ketonic, carboxylic and amino (primary) groups. |
| J. Study of carbohydrates, fats and proteins in pure form and detection of their presence in given food stuffs. |
| K. Qualitative analysis: Determination of one catiop and one anion in a given salt.
Cations– Pb 2+, Cu+2, As3+, AI3+, Fe3+, Mn2+, Zn2+, Co2+, Ni2+., Ca2+, Sr2+, Ba2+, Mg2+., NH4+ Anions- CO32-, S-2, SO32-, NO2-1, NO3-1,Cl–, Br– , I-1, PO43-., C2O4 2-, CH2COO– , NO (Note: Insoluble salts excluded) |
PROJECT
Scientific investigations involving laboratory.
Testing and collecting information from other sources.
A few suggested Projects
1. Study of presence of oxalate ions in guava fruit at different stages of ripening.
2. Study of quantity of casein present in different samples of milk.
3. Preparation of soyabean milk and its comparison with the natural milk with respect to curd formation, effect of temperature etc.
4. Study of the effect of potassium bisulphate as food preservative under various conditions (temperature, concentration, time etc,)
5. Study of digestion of starch by salivary amylase and effect of PH and temperature on it.
6. Comparative study of the rate of fermentation of following material wheat flour. gram flour, Potato juice, carrot juice etc.
7. Extraction of essential oils present in saunf (aniseed), Ajwain (carum) illaichi (cardamom).
8. Study of common food adulterants in fat, oil, butter, sugar, turmeric powder, chilli powder and pepper.
Note: Any investigatory project, which involves about 10 periods of work, can be chosen with the approval of the teacher.
Students can also find more free study material like sample papers, previous year papers and PSEB Textbooks from BYJU’S.
Comments