Sulfate Ion formula, also known as Sulfate anion formula or Sulfate dianion formula is explained in this article. It is a polyatomic anion and has an empirical formula SO4-2. It is a salt of sulfuric acid. The sulfate anion is a very weak base.
Most of the sulfates such as Na+, NH4+and K+ are soluble in water whereas white barium sulfate and white lead (II) sulfate are insoluble in water. It is a weak oxidizing agent and is obtained by deprotonation of OH groups of sulfuric acid (H2SO4). Sulphate salts, peroxides, and acid derivatives of sulfate have wide applications in industries.
Sulfate Ion Formula Structure
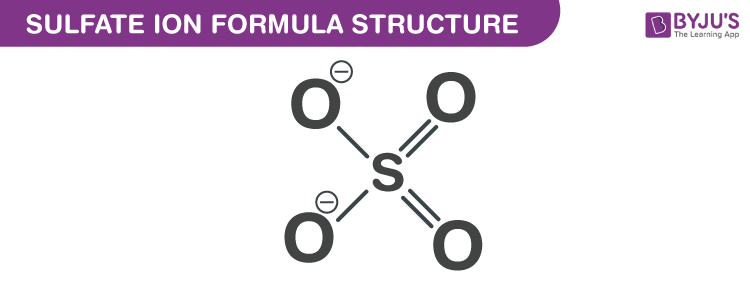
Properties Of Sulfate Ion
| Chemical formula | SO4-2 |
| Molecular weight | 96.06 g/mol |
| Conjugate acid | Hydrogen sulfate |
| Boiling point | 623.89 °C |
| Melting point | 270.47 °C |
Metal sulfates can be prepared by treating metal, metal oxide, or metal hydroxide with sulfuric acid (H2SO4). Or by oxidizing metal sulfites or sulfides.
To learn more about Sulfate Ion formula from the expert faculties at BYJU’S, register now!

Comments