Tin (IV) Chloride, also known as Stannic Chloride, is an inorganic compound with a chemical formula SnCl4. It is hygroscopic and fumes when it comes in contact with air. It can be prepared from the reaction of tin with chlorine gas at 115 °C. In this short piece of article, learn more about the tin (IV) chloride formula, its chemical structure along with its properties and uses.
Tin (IV) Chloride Properties
| Properties of Tin (IV) Chloride | |
| Name | Tin (IV) Chloride |
| Other Names | Stannic Chloride, Tin tetrachloride, Tetrachlorostannane |
| Appearance | Colourless to slightly yellow fuming liquid |
| Chemical Formula | SnCl4 |
| Melting Point | -33 °C (anhydrous)
56°C (pentahydrate) |
| Boiling Point | 114 °C |
| Molar Mass | 260.5 g/mol |
| Density | 2.226 g/cm3 (anhydrous)
2.04 g/cm3 (pentahydrate) |
| Solubility in water | Soluble |
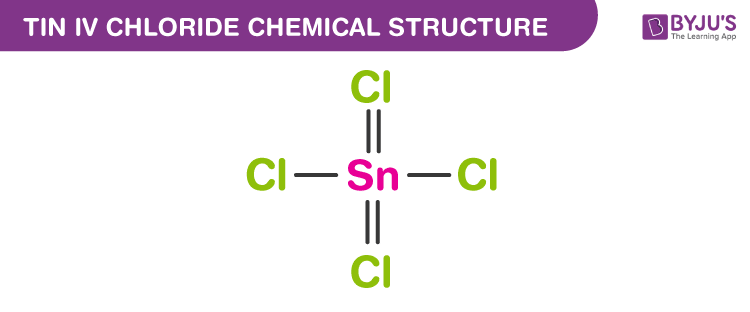
Tin (IV) Chloride Chemical Structure
Tin (IV) Chloride Uses
- Used as catalysts and polymer stabilizers
- Used in the preparation of SnO2 coating for toughening glass
- Used as a precursor to other tin compounds
Stay tuned to BYJU’S to know more about various chemical compounds along with in-depth details.
Comments