West Bengal Madhyamik Board 10th Standard Physical Science Exam Question Paper 2017- Free Download
The West Bengal Madhyamik Board Class 10 Physical Science 2017 Question Paper is the best resource for the students who are preparing for the Class 10 board exams. This previous year paper sets the foundation for the question types that are likely to be asked in the Physical Sciences exam. Students can solve and practise the West Bengal Madhyamik Board Class 10 Physical Science 2017 Question Paper to get an idea on how to answer the questions most effectively.
Madhyamik Students can access the question paper from the respective link and practise the unsolved question paper PDF. Students need to only click on the interactive PDF link to get the printable format of the question paper. The questions and answers are published online on the web-page, as well.
Madhyamik Students are also urged to get acquainted with the main topics, which are covered in the West Bengal Board Class 10 Physical Science textbook. The below given question paper, in the meantime is the perfect resource for students. It helps them to get an overview of the question paper format of the WBBSE Class 10 Physical Science exam. Students are advised to practise the previous year papers of West Bengal Board Class 10 Physical Science in order to revise the subject thoroughly. Referring to these previous year question papers will also help the students to gauge their performance and preparation level for the board exams. The students can solve these papers regularly to know how to answer the questions effectively.
Download West Bengal Madhyamik Board 2017 Physical Science Question Paper
West Bengal Madhyamik Board Class 10 Physical Science 2017 Question
GROUP-A
- MCQ type Questions. Four options are given as answers to each of the following questions. Select and write the correct answer. [1×15=15]
1.1 Which among the following is not a greenhouse gas?
(a) methane
(b) water vapour
(c) carbon dioxide
(d) oxygen
1.2 Which of the following is the SI unit of pressure?
(a) Nm2
(b) Nm-2
(c) Nm
(d) N
1.3 If the vapour density of a gas is 32, which among the following is its molecular weight?
(a) 8
(b) 16
(c) 32
(d) 64
1.4 On which of the following does the thermal conductivity of a metal depend?
(a) temperature
(b) length
(c) nature of the material
(d) area of the cross-section
1.5 What is the relation between the radius of curvature and the focal length of a mirror?
- f=2r
- f=r/2
- f=r/3
- f=(⅔) r
1.6 Which of the following colours deviates most when white light is refracted by a prism?
(a) red
(b) yellow
(c) violet
(d) green
1.7 Which of the given physical quantities represents ampere?
(a) coulomb-second
(b) volt-ohm-1
(c) volt-ohm
(d) volt-1 ohm
1.8 Resistivity of which of the following decreases with increase in temperature?
(a) conductor
(b) semiconductor
(c) superconductor
(d) insulator
1.9 Which of the following is the correct order of ionising power for α, β and γ rays?
(a) α >β > γ
(b) α > γ > β
(c) γ > β > α
(d) β > α > γ
1.10 Which of the following has the highest atomic radius?
(a) K
(b) H
(c) Li
(d) Na
1.11 In which of the following is a covalent bond present?
(a) hydrogen chloride
(b) sodium chloride
(c) lithium hydride
(d) calcium oxide
1.12 Which of the following is a weak electrolyte in aqueous solution?
(a) CH3COOH
(b) NaOH
(c) H2SO4
(d) NaCl
1.13 Which of the following is used to dry moist ammonia?
(a) conc. H2SO4
(b) P2O5
(c) CaO
(d) CaCl2
1.14 Which of the following is an ore of aluminium?
(a) bauxite
(b) haematite
(c) malachite
(d) chalcopyrites
1.15 Which of the following is the functional group of aldehydes?
- -OH
- -OH
- >C=O
- -COOH
GROUP-B
2. Answer all the questions (options are given for some questions)
2.1 Fill in the blanks
In troposphere temperature ______ with increase in altitude
Or
Mention one harmful effect of global warming.
2.2 Which atom, released from a chlorofluorocarbon under the influence of UV rays, decomposes ozone into oxygen?
2.3 Write whether the following statement is true of false:
Under the same condition of temperature and pressure equal volumes of CO2 and N2 gas contain a different number of molecules.
2.4 Write down the relation between the pressure and volume of a given mass of a gas, according to Boyle’s law when temperature remains constant.
2.5 Between the apparent and real expansion coefficients of a liquid, which one is its own characteristic?
Or
What is the SI unit of thermal conductivity?
2.6 Give a natural example of dispersion of light.
2.7 Which type of lens can be used to rectify myopic vision?
2.8 What will be the change in the motion of a Barlow’s wheel if the direction of the current is reversed?
2.9 How much work has to be done when 1 coulomb of charge is taken against a potential difference of 1 volt?
2.10 From which part of a radioactive atom is a β- particle emitted?
Or
What is the atomic number of the daughter element produced if an α -particle is emitted from 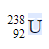 ?
?
2.11 Match the right column with the left column [1×4]
| Left Column | Right Column |
| 2.11.1 A transition element | (a) Aluminium |
| 2.11.2 A transuranium elements | (b) Nickel |
| 2.11.3 Reduces Fe2SO3 at high temperature in the thermit process | (c) Tin |
| 2.11.4 Present in the alloy bell metal | (d) Plutonium |
2.12 Draw Lewis dot diagram of F2 molecule. (F=9)
2.13 Why is the electrical conductivity of the solution produced by adding little sulphuric acid into pure water more than the electrical conductivity of pure water?
Or
What are the carriers of electricity in the solution during the electrolysis of aqueous solution of an electrolyte?
2.14 Which ion migrates towards the cathode during the electrolysis of an aqueous solution of CuSO4, using Cu electrodes?
2.15 Write down the formula of the black precipitate formed when H2S gas is passed through an aqueous solution of lead nitrate.
Or
What changes of colour is observed when H2S gas is passed through an acidified aqueous solution of potassium dichromate?
2.16 Two substances are used in the production of urea. One is ammonia, what is the other?
2.17 Mention one use of poly (vinyl chloride).
2.18 Write down the IUPAC name of CH3CH2CH2OH.
Or
Write down the constitutional formula of an isomer of CH3CH2OH.
GROUP-C
3. Answer all the given questions.(Alternatives are given for some). [2×9=18]
3.1 What is meant by calorific value of a fuel? Between coal and diesel, which has greater calorific value?
3.2 If the volume of a given mass of a gas at STP is 52 m3, what will be its volume at the pressure of 104 cmHg temperature remaining constant?
Or
What will be the volume of 8g of H₂ gas(H=1) at the pressure of 4 atmosphere and temperature of 300K? (R= 0.082 litre atmosphere mol-1 K-1)
3.3 How does the refractive index of a medium depend on the velocity of light?
Or
What kind of mirror is used in the headlight of a car and why?
3.4 Mention two advantages of using a CFL lamp over an incandescent lamp?
3.5 An aqueous solution of sugar cannot conduct electricity, but an aqueous solution of sodium chloride can. Why?
Or
Why is it appropriate to use the term formula weight instead of molecular weight in case of sodium chloride?
3.6 Compare the following two properties of naphthalene and sodium chloride: melting point and solubility in water.
3.7 Name a gas which can be prepared in Kipp’s apparatus. Write the balanced chemical equation of the reaction for the preparation of the gas.
3.8 How is metallic zinc obtained from zinc oxide? Write the reaction with a balanced chemical equation.
Or
What happens when a piece of zinc is added to an aqueous solution of CuSO4? Show with the help of electronic theory that this is an oxidation-reduction reaction.
3.9 Show with the help of structural formula of ethylene that it is an unsaturated hydrocarbon.
Or
What is a denatured spirit?
GROUP-D
4. Answer the given questions (Alternative questions are provided for some)
4.1 State the Charles’ law related to gases and represent it graphically ( V vs t)
4.2 How many grams of ammonium sulphate will be required to prepare 6.8g of ammonia by heating ammonium sulphate with caustic soda solution?
(H=1, N=14, O=1, S=32)
Or
CaCl2, CO2 and H2O are produced by the reaction of dilute HCl with CaCO3. How many grams of HCl will be required to prepare 55.5g of CaCl2, 22.2 g of CO2 and 9.0 g of H2O from 50.0g of CaCO2? What is the number of mole of HCl required? [H=1, Cl=35.5]
4.3 What is the volume expansion coefficient of a gas at a fixed pressure? What is its value?
Or
Mention two similarities between heat conduction and electrical conduction. Mention a non-metal having high thermal conductivity.
4.4 State the two laws of refraction of light. Also write the mathematical formula of the second law.
4.5 In case of a concave mirror, find the relationship between focal length and radius of curvature.
Or
A ray of light passes through a prism. Show the angle of deviation δ = i1 + i2 – A. (Symbols have usual meaning)
4.6 Write Faraday’s laws of electromagnetic induction. What is meant by alternating current?
4.7 An electric bulb of resistance 440 ohm is connected to 220 volt mains for 10 hours. Find the amount of electrical energy consumed in BOT unit.
Or
A metal wire of resistance 6 ohm is stretched so that its length becomes double its original length. What would be the final resistance?
4.8 What is meant by mass defect? What is the source of energy released in nuclear fusion?
4.9 Write with an example what is meant by periodic property of elements? Mention a property which is not periodic.
Or
What is meant by electronegativity of an element? How does electronegativity of Group 1 elements of the long periodic table change from top to bottom?
4.10 Which energy is responsible for the chemical reaction to occur during electrolysis? What reactions do occur at the cathode and the anode during electrolysis of acidulated water?
4.11 Write a balanced chemical equation with conditions for the industrial manufacture SO3 from SO2 by the contact process. How is H2SO4 prepared from the SO3 produced?
4.12 Write balanced chemical equation of addition reaction of hydrogen with acetylene mentioning the conditions of reaction. Mention one use of LPG.
Or
How do you convert CH3CH2OH to CH2=CH2?
How is biopolymer protein biodegraded in nature?
GROUP – E
(Only for External Candidates)
5. Answer any four of the following questions: [1×4=4]
5.1 What is the main component of biogas?
5.2 What is the SI unit of electric charge?
5.3 How many elements are there in the third period of the long periodic table?
5.4 What is the radioactive ray that is not made up of particles? What is the industrial source of CNG?
6. Answer any three of the following questions. [2×3=6]
6.1 What is the absolute scale of temperature?
6.2 Mention two advantages of AC over DC.
6.3 Write with the balanced chemical equation what happens when an aqueous solution of ammonia is added to an aqueous solution of ferric chloride.
6.4 Write with the balanced chemical equation what happens when methane undergoes combustion in oxygen.
Thedefinite articleMore (Definitions, Synonyms, Translation)





Comments