Zinc Sulfate formula, also known as White Vitriol formula or Zincate formula is explained in this article. Its molecular mass in monohydrate form is 179.47 g/mol and heptahydrate form is 287.53 g/mol. The chemical or molecular formula of Zinc Sulfate is ZnSO4.
White Vitriol is obtained as a colourless crystalline solid or white powder. It is soluble in water and alcohols. Anhydrous Zincate is a crystalline solid which has no colour. It is a non-combustible compound. It is widely used in the production of rayon, as a fertilizer ingredient, as a dietary supplement to treat zinc deficiency. Also, it is widely used as an astringent in eye drops and lotions.
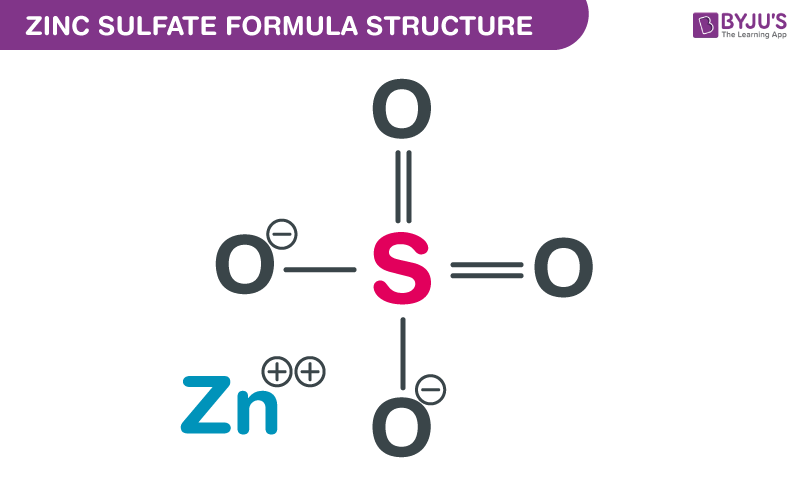
Zinc Sulfate Formula Structure
Properties Of Zinc Sulfate Formula
| Chemical formula | ZnSO4 |
| Molecular weight | 161.47 g/mol (anhydrous) |
| Density | 3.54 g/cm3 |
| Boiling point | 740 °C |
| Melting point | 680 °C |
White Vitriol powder is an eye irritant. Ingesting trace amounts of this inorganic compound is considered safe. Therefore, ZnSO4 is added to animal feed as a source of essential zinc. Excess consumption may cause acute stomach distress leading to nausea as well as vomiting.
To learn more about Zinc Sulfate formula from the expert faculties at BYJU’S, register now!

Comments