Aluminium carbonate is a carbonate of an aluminium salt with a chemical formula Al2(CO3)3, that doesn’t exist in normal conditions. It can be manufactured with the high pressure of carbon dioxide and temperature close to 0°C. For aluminium carbonate storage, one would have to create a very complex system that would protect the substance against any external factors. In this short piece od article, learn more about the aluminium carbonate formula and its chemical structure along with the aluminium carbonate properties and uses.
Aluminium Carbonate Properties
| Properties of Aluminium Carbonate | |
| Name | Aluminium Carbonate |
| Appearance | White Powder |
| Molecular Formula | Al2(CO3)3 |
| Melting Point | 58 °C |
| Boiling Point | Decomposes |
| Density | 1.5 g/cm³ |
| Molar Mass | 96.09 g/mol |
| Solubility in Water | Soluble in water |
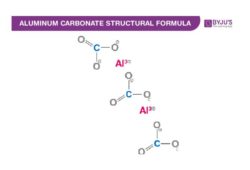
Aluminium Carbonate Chemical Structure
Aluminium Carbonate Uses
- Used as a phosphate-binding drug that is administered to cats and dogs
- Used as a drug to treat stomach inflammation and ulcerations
- It is taken to prevent the formation of urinary stones in humans.
To learn more about such chemistry topics register to BYJU’S now!

Comments