Iron (III) hydroxide is a chemical compound made of Iron, hydrogen and oxygen with a chemical formula Fe(OH)3. Depending on the hydration, crystal structure and particle shape and size, the colour of iron III hydroxide varies from dark-brown to black. In this short piece of article, you will learn more about the Iron III hydroxide formula, its properties, chemical structure and uses.
Iron III Hydroxide Properties
| Properties of Iron III Hydroxide | |
| Name | Iron III Hydroxide |
| Also known as | Ferric acid |
| Appearance | Vivid dark orange crystals |
| Chemical Formula | Fe(OH)3 |
| Melting Point | 135 °C |
| Density | 4.25 g/cm³ |
| Molar Mass | 106.867 g/mol |
| Solubility in Water | Insoluble |
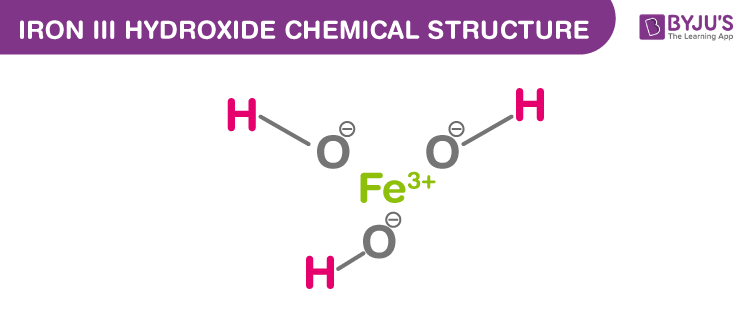
Iron III Hydroxide Chemical Structure
Iron III Hydroxide Uses
- Yellow iron oxide is used in a few cosmetics, tattoo ink
- Used in aquarium water treatment as a phosphate binder
- Used as possible adsorbents for lead removal from aquatic media
To learn more about such chemistry topics register to BYJU’S now!

Comments