It is a pale violet crystalline, a non-flammable chemical compound. It forms a yellow solution when dissolved due to a process called hydrolysis. It is used by metalsmiths and jewellers to etch silver and silver alloys. It is also used in the process like dyeing, chemical analysis, tanning, and in the production of medicine.
Properties Of Iron (III) Nitrate
| Chemical formula | Fe(NO3)3 |
| Molecular weight | 241.857 g/mol (Anhydrous)
403.999 g/mol (nonahydrate) |
| Density | 1.68 g/cm3 (hexahydrate)
1.6429 g/cm3(nonahydrate) |
| Chemical names | Ferric nitrate, Nitric acid, and iron(3+) salt |
| Boiling point | 125 °C |
| Melting point | 47.2 °C |
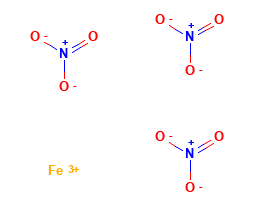
Iron (III) Nitrate Structural Formula
The structural representation of Iron (III) Nitrate is as shown in the figure below. Iron III Nitrate compound is obtained when the iron metal powder is treated with nitric acid.
Fe + 4 HNO3 → Fe(NO3)3 + NO + 2 H2O.
To learn more about various chemical compounds and facts stay tuned with BYJU’S!!

Comments