Iron (III) Sulfate formula or the ferric sulfate is an inorganic salt with the formula Fe2(SO4)3. It is yellow in colour and is soluble in water. The molecule is formed of Fe+3 cation and SO4–2 anion. It is found in a wide variety of minerals but mainly it is found in marcasite and pyrite. Mostly, ferric sulfate is extracted from nature but can also be prepared by treating ferrous sulfate with sulfuric acid at elevated temperature. In this article, learn more about the Iron (III) Sulfate formula and its chemical structure along with its properties and uses.
Iron (III) Sulfate Properties
| Properties of Iron (III) Sulfate | |
| Name | Iron (III) Sulfate |
| Also Known as | Ferric Sulfate, Sulfuric acid – iron(3+) salt |
| Appearance | Yellow crystalline solid or greyish-white powder. |
| Chemical Formula | Fe2(SO4)3 |
| Melting Point | 480 °C (anhydrous)
175 °C (nonahydrate) |
| Density | 3.1 g/cm³ (anhydrous)
1.9 g/cm3 (pentahydrate) |
| Molar Mass | 399.88 g/mol(anhydrous)
486.96 g/mol (pentahydrate) 562.00 g/mol (nonahydrate) |
| Solubility in Water | Slightly Soluble |
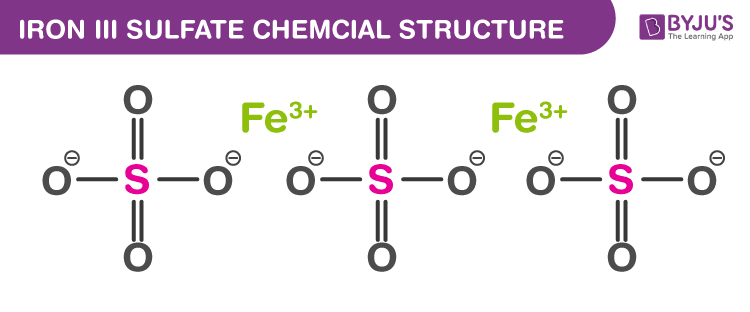
Iron (III) Sulfate Chemical Structure
Iron (III) Sulfate Uses
- Used in water treatment processes to eliminate the sulfur odour
- Used as an oxidizing agent and solid separation agent
- Used in medicines as astringent and styptic.
To learn more about such chemistry topics register to BYJU’S now!
Comments