Magnesium acetate exists in anhydrous and hydrous form. In anhydrous form, it possesses a chemical formula Mg(C2H3O2)2 and in the hydrous form, it possesses a chemical formula Mg(CH3COO)2 • 4H2O. Magnesium acetate is a safe compound to handle and it has a zero health hazard rating. However, it should be handled with care using gloves and goggles because it can cause mild irritation at the point of contact. In this article, learn about the magnesium acetate formula, its properties and chemical structure along with its uses.
Magnesium Acetate Properties
| Properties of Magnesium Acetate | |
| Name | Magnesium Acetate |
| Appearance | White Hygroscopic crystals |
| Chemical Formula | Mg(C2H3O2)2 |
| Melting Point | 80 °C |
| Density | 1.45 g/cm3 |
| Molar Mass | 142.394 g/mol |
| Solubility in Water | Soluble |
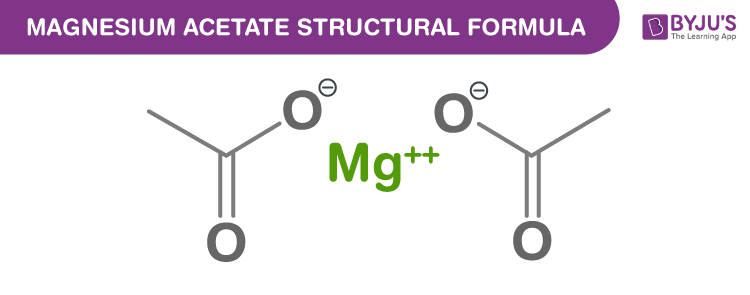
Magnesium Acetate Chemical Structure
Magnesium Acetate Uses
- Used in the dyeing of ceramic tiles and textiles
- Used as a catalyst in the polyester film production
- Used as a dialysis solution
- Used in offset printing
Storage And Safety Measurements
- Since magnesium acetate absorbed moisture it should be placed away from the water.
- Magnesium acetate should not mix with oxidizers since they are incompatible.
- Magnesium acetate is health hazards compound should always handle with gloves and goggles.
To learn more about such chemistry topics register to BYJU’S now!

Comments