Mercury (II) sulfate formula, also named as Mercuric sulfate formula or Mercury Bisulfate formula is discussed in this article. In the year 1932, it was used as a catalyst to produce acetaldehyde from water and acetylene. The molecular or chemical formula of Mercury (II) sulfate is HgSO4.
It is an odourless monoclinic crystal. It also occurs in the form of white crystalline powder or granule. Mercuric sulfate can be prepared by heating concentrated sulphuric acid with elemental mercury (Hg). It is soluble in hot sulphuric acid, sodium chloride solution and insoluble in ammonia, alcohol, and acetone.
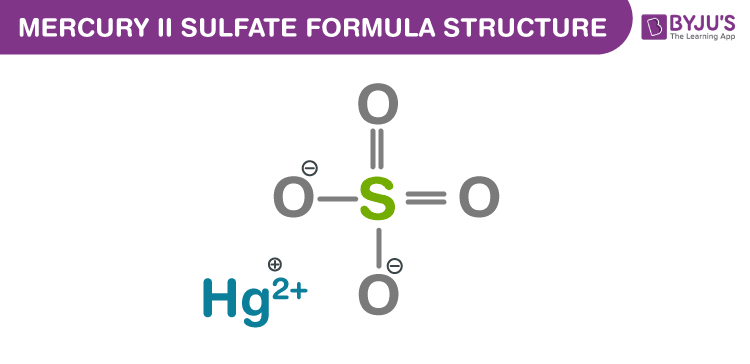
Mercury (II) sulfate Formula Structure
Properties Of Mercury (II) sulfate Formula
| Chemical formula | HgSO4 |
| Molecular weight | 296.653 g/mol |
| Density | 6.47 g/cm3 |
| Sublimation conditions | 450 °C |
Safety Measures
- Inhaling this compound causes acute poisoning which leads to tightness in the chest, coughing, and difficulties breathing.
- When it is exposed to eyes it may cause ulceration of conjunctiva.
- If this compound comes in contact with skin it causes dermatitis.
- Ingesting this compound will cause death as a result of the peripheral vascular collapse.
To learn more about Mercury (II) sulfate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Mercury Bisulfate for free.

Comments