Sodium Thiosulfate is an inorganic compound that has a chemical formula Na2S2O3. It consists of two sodium (Na+) cations and a positively charged thiosulfate ion (S2O3–). Sodium thiosulfate can be prepared by heating sulfur with sodium sulfite solution.
\(\begin{array}{l}6NaOH+4S\rightarrow Na_2S_2O_3+2Na_2S+3H_2O\end{array} \)
The other names of sodium thiosulfate are sodium hyposulfite, hyposulphite of soda. In this short piece of article, we will be looking more into the sodium thiosulfate formula, its chemical structure along with its properties and uses.
Sodium Thiosulfate Properties
| Properties of Sodium Thiosulfate | |
| Name | Sodium Thiosulfate |
| Also Known as | Thiosulfuric acid, Sodium Hyposulfite |
| Appearance and odour | White crystals
Odour – Odourless |
| Molecular Formula | Na2S2O3 |
| Melting Point | 48.3 °C (pentahydrate) |
| Boiling Point | 100 °C (pentahydrate) |
| Density | 1.67 g/cm³ |
| Molar Mass | 158.11 g/mol (anhydrous)
248.18 g/mol (pentahydrate) |
| Solubility in Water | Soluble |
Sodium Thiosulfate Chemical Structure
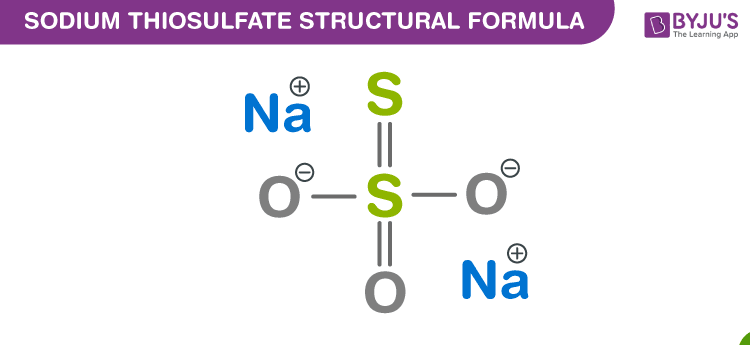
Sodium Thiosulfate Uses
- Used in several pharmaceutical products
- Used in gold extraction
- Its application also lies in leather tanning, photographic processing and water treatment
To learn more formulas of various chemical compounds, subscribe to BYJU’S!

Comments