Ammonium sulfide, also known as the “stink bomb,” is a toxic chemical and has a very strong and unpleasant odour. They are highly flammable and the ammonium sulfide molecular formula is represented as follows (NH4)2S.
Treating ammonium hydroxide with an excess of hydrogen sulfide, ammonium hydrosulfide (NH4HS) is formed, which with further treatment with the same quantity of ammonia yields ammonium sulfide. In this article, we will be discussing the ammonium sulfide formula and its general properties.
Ammonium Sulfide Properties
| Ammonium Sulfide Properties | |
| Name | Ammonium Sulphide |
| Also Known as | Stink Bomb |
| Appearance | Yellow crystal |
| Chemical Formula | (NH4)2S or N2H8S |
| Molar Mass | 66.13 g/mol |
| Density | 0.997 g/cm3 |
Ammonium Sulfide Structural Formula
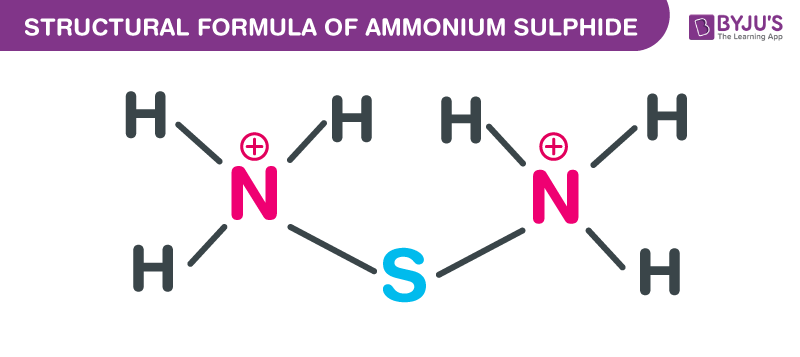
Ammonium Sulfide Uses
- Used in the manufacture of photographic developers
- Used in the textile industry
- Used in the application of the patina to the bronze
To learn more about such chemistry topics register to BYJU’S now!
Comments