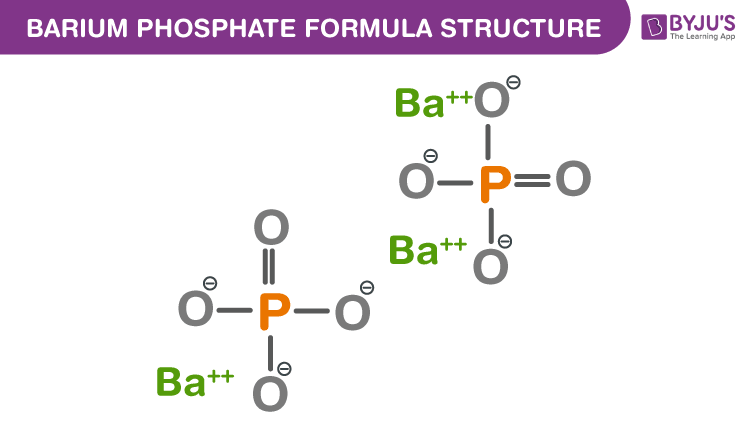
Barium phosphate formula, also known as Tribarium diphosphate formula or Barium phosphate tribasic formula is explained in this article. It is an inorganic salt consists of 3 cations and 2 anions. The cation is barium (Ba2+) and the anion is phosphate (PO43-). The chemical or molecular formula of Barium phosphate is Ba3(PO4)2.
Barium phosphate tribasic is colourless solid which usually occurs in its powder form. which has a slight odour of acetic acid. It does not dissolve in water but readily dissolves in acidic liquid solutions. Tribarium diphosphate is found in rocks and minerals such as alforsite and jagowerite. It can be synthesized by reacting inorganic salt barium carbonate with the metaphosphoric acid (HPO3).
Barium Phosphate Formula Structure
Properties Of Barium phosphate
| Chemical formula | Ba3(PO4)2 |
| Molecular weight | 295.27 g/mol |
| Density | 3.63 g/mL |
| Complexity | 36.8 |
| Melting point | 1560°C |
Use And Safty Measure Of Barium phosphate
- It is widely used in the production of the pulsed laser and glasses.
- It is inflammable.
- Inhaling or swallowing this compound is dangerous to health.
- It can cause eye and skin irritation.
To learn more about Barium phosphate formula from the expert faculties at BYJU’S, register now!

Comments