Copper(II) Chloride formula, also known as Cupric chloride formula or Copper dichloride formula is explained in this article. It is an inorganic chloride of copper and the oxidation state of the metal is +2. The molecular or chemical formula of Copper(II) Chloride is CuCl2.
In its anhydrous form, it appears as a yellowish-brown powder whereas in its dihydrate form it appears as a green crystalline solid. It is non-combustible but there are chances of formation of hydrogen chloride gas when heated. It is corrosive to aluminium. It is widely used in the manufacturing of various chemicals, in printing, in dyeing, as a wood preservative, and in fungicides.
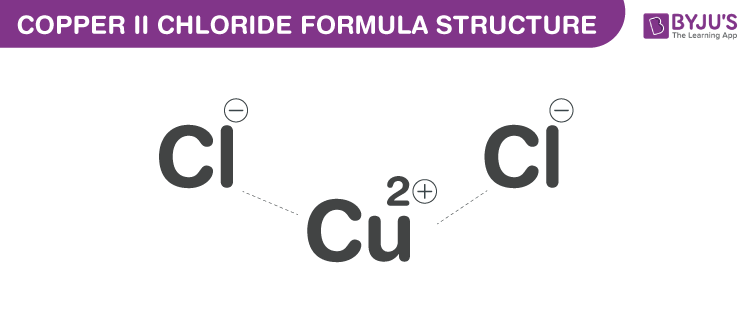
Copper(II) Chloride Formula Structure
Properties Of Copper(II) Chloride
| Chemical formula | CuCl2 |
| Molecular weight | 134.45 g/mol (anhydrous)
170.48 g/mol (dihydrate) |
| Density | 3.386 g/cm3 (anhydrous)
2.51 g/cm3 (dihydrate) |
| Boiling point | 993 °C (anhydrous) |
| Melting point | 498 °C (anhydrous)
100 °C (dehydration of dihydrate) |
Copper dichloride in the form of injection is considered a nonpyrogenic, sterile solution recommended for the use for Total Parenteral Nutrition.
To learn more about Copper(II) Chloride formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Cupric Chloride for free.

Comments