Magnesium Sulfide is brown in colour and is crystalline in nature. It is formed when sulfur or hydrogen sulfide reacts with magnesium. It is found in the circumstellar envelopes of certain evolved carbon stars and meteorites. Let’s know the chemical composition of Magnesium Sulfide.
Properties Of Magnesium Sulfide
| Chemical Formula | MgS |
| Molecular Weight | 56.365 g/mol |
| Chemical Name | Niningerite |
| Melting Point | 2,000 °C |
| Solubility in water | Decomposes |
Magnesium Sulfide Structural Formula
Magnesium Sulfide contains magnesium metal cation with Mg+2 charge on it and nonmetal Sulfur anion with S−2 charge on it. It is a cubic in nature crystalline structure that appears white to reddish-brown in colour.
It will take one -2 sulfide ion to balance one +2 magnesium ion to form a magnesium sulfide.
The structural formula of Magnesium sulfide is as shown below
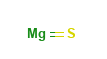
For more such interesting information, visit BYJU’S!

Comments