Both nitrification and denitrification are the different stages involved in the biogeochemical process of the nitrogen cycle.
What is Nitrification?
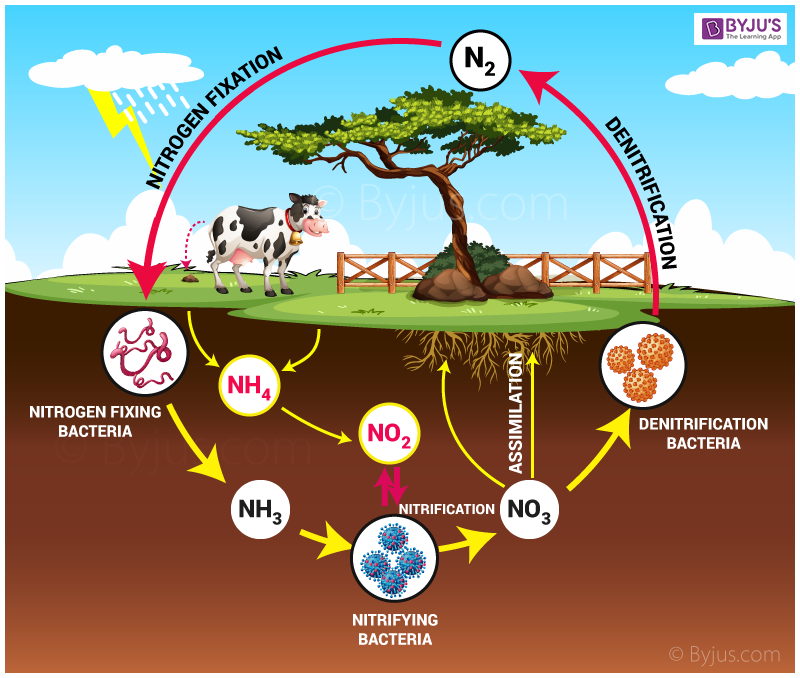
Nitrification is the natural process that occurs in the environment by a group of specialised bacteria during which (NH4+) ammonia is converted to (NO2–) nitrites and then to (NO3–)nitrates. Nitrosomonas are a group of specialised bacteria involved in converting ammonia into nitrates. Nitrosomonas is a genus of gram-negative rod-shaped chemoautotrophic bacteria. This is the first and essential stage of the nitrogen cycle. The overall reaction of nitrification is:
NH4+(Ammonium) → NO2– (Nitrite) → NO3– (Nitrate).
Explore more: Nitrogen Cycle
What is Denitrification?
Denitrification is a biological process of reduction in which the nitrogen compounds are released back into the atmosphere in their gaseous form – nitrogen gas (N) by the conversion of nitrate (NO3–). Therefore, this is the final stage of the nitrogen cycle, carried on without oxygen. The entire process of denitrification is carried out by the denitrifying bacterial species- Clostridium and Pseudomonas. The overall reaction of denitrification is:
2NO3− (Nitrate) + 10e− + 12H+ → N2 (Nitrogen) + 6 H2O (Water molecules).
Explore more: Denitrification.
Let’s learn the differences between nitrification and denitrification.
Nitrification and Denitrification – Comparisons
| Definition | |
| This is a biological process of converting ammonium (NH4+) into nitrate (NO3–). | This is a biological process of converting nitrate (NO3–) into nitrogen gas (N2). |
| Involvement of Bacteria | |
| Nitrosomonas and Nitrobacter. | Lactobacillus, Spirillum, Pseudomonas, and Thiobacillus. |
| Growth and Development | |
| The growth is prolonged. | The growth is fast. |
| Mode of Respiration | |
| Aerobic mode of Respiration. | Anaerobic mode of Respiration. |
| Mode of Nutrition | |
| Autotrophic. | Heterotrophic. |
| Precursors | |
| Ammonium (NH4+) | Nitrate (NO2– ) |
| By-Product | |
| Nitrate (NO2– ) | Nitrogen (N) |
| pH | |
| An optimal pH is between 6 to 8. | An optimal pH is between 6 to 8. |
| Temperature | |
| The minimum temperature required for this process is between 16° and 35°C. | The minimum temperature required for this process is between 26° and 38°C. |
| Applications | |
| Nitrate, the by-product of the nitrification process, is rich in nitrogen and is a rich source for plant growth and development. | This is the best process used in wastewater treatment by removing nitrogen from sewage and other municipal wastewater. This biological process also supports aquatic life. |
Also Refer: Difference Between Biomagnification and Bioaccumulation.
This article concludes with an introduction to nitrification and denitrification and their differences.
Stay tuned to BYJU’S Biology to learn more in detail about the nitrogen cycle, its process, significances, and also more similarities and differences between nitrification and denitrification.
Comments