Potassium hypochlorite with the formula KClO is a pungent colourless solution with an irritating odour. Potassium hypochlorite can be prepared by the reaction of chlorine with potassium hydroxide. It can also be produced by the electrolysis of potassium chloride solution. In both methods, the reaction mixture must be kept cold to prevent the formation of potassium chlorate. In this article, we shall learn more about the potassium hypochlorite formula, its chemical structure, properties and uses.
Potassium Hypochlorite Properties
| Properties of Potassium Hypochlorite | |
| Name | Potassium Hypochlorite |
| Appearance and odour | Light grey liquid
Odour – Chlorine like |
| Chemical Formula | KClO |
| Melting Point | −2 °C |
| Boiling Point | 102 °C |
| Density | 1.160 g/cm3 |
| Molar Mass | 90.55 g/mol |
| Solubility in Water | Slightly soluble |
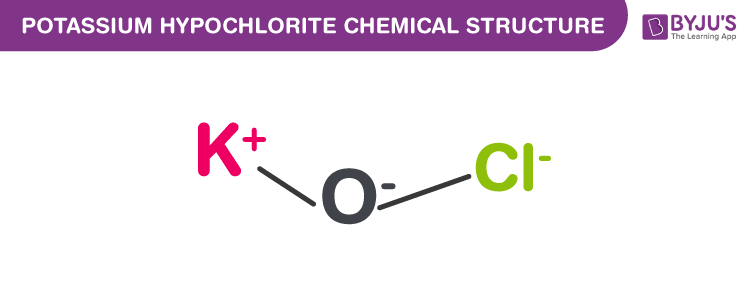
Potassium Hypochlorite Structure
Potassium Hypochlorite Uses
- Used for disinfecting water and sanitizing the surface
- Used as a bleaching agent
- Used as an industrial oxidizer
- Used in agriculture field
To learn more about such chemistry topics register to BYJU’S now!

Comments