Science is a subject in which a student can score full marks if they practice diligently. One valuable resource that can provide students with the required practice is the Maharashtra SSC Board Science Previous Year Question Papers. MSBSHSE Class 10 Science is of two parts, and here we provide the Maharashtra SSC Board Solved Previous Year Question Papers of Science Part 1 and Part 2.
These question papers will give students a general idea about the exam paper pattern and the type of questions to expect from a particular topic. Meanwhile, students can also refer back to these solved question papers with answers given to get an idea on how to write the best answers.
| Download Science Part 1 Question Paper 2019 |
| Download Science Part 2 Question Paper 2019 |
MSBSHSE Class 10 Science Part 1 Questions with Solutions
1) (A) Answer the following Questions: (5)
1. Write the proper answer in the box:

If F = Gm1m2/ d2, then F=
Answer: Gm1m2/ 9d2
As given in the second figure, distance is 3d. So, replacing the value in the formula:
F= Gm1m2/ (3d) 2 = Gm1m2/ 9d2.
2. In Dobereiner’s triads Li, Na, K, the atomic masses of Lithium and Potassium are 6.9 and 39.1, respectively. What will be the atomic mass of sodium?
Answer: The Law of Dobereiner’s Triads states that the atomic mass of Na is the average of the atomic masses of Li and K. Hence, Atomic mass of Sodium (Na) = (6.9 + 39.1)/ 2 = 23.
3. State whether the given statement is true or false:
A concave lens is a converging lens.
Answer: False.
When the refracted rays through the lens are converged at one point, it is called converging lens. However, concave lens spread the light that is refracting through it. Hence, a concave lens is a diverging lens.
4. By considering first correlation, complete the second correlation:
Hubble telescope: 569 km high from earth surface
Revolving orbit of Hubble telescope:
Answer: Low Earth Orbit
If the height of the satellite orbit above the earth’s surface is in between 180 km and
2000 km, the orbits are called Low Earth Orbits. Hence, the revolving orbit of Hubble telescope is Low Earth Orbit.
5. Find the odd man out:
Tinning, Anodization, Alloying, Froth floatation
Answer: Froth Floatation
Tinning, Anodization and Alloying are the processes of coating a thin layer of metal on the surface of other metals. While, Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic and is used in mineral processing, paper recycling and waste-water treatment industries.
(B) Choose the correct alternative: (5)
1. The reaction of iron nail with copper sulphate solution is _______ reaction.
(A) Combination
(B) Decomposition
(C) Displacement
(D) Double displacement
Answer: (C) Displacement
Iron being more reactive than copper can displace copper from its compounds/salts such as copper sulphate solution. Hence, the displacement reaction happens and blue colour of copper sulphate is altered to light green colour of iron sulphate.
Given below is the reaction :->
Fe + CuSO4 (aqueous) –> FeSO4 + Cu
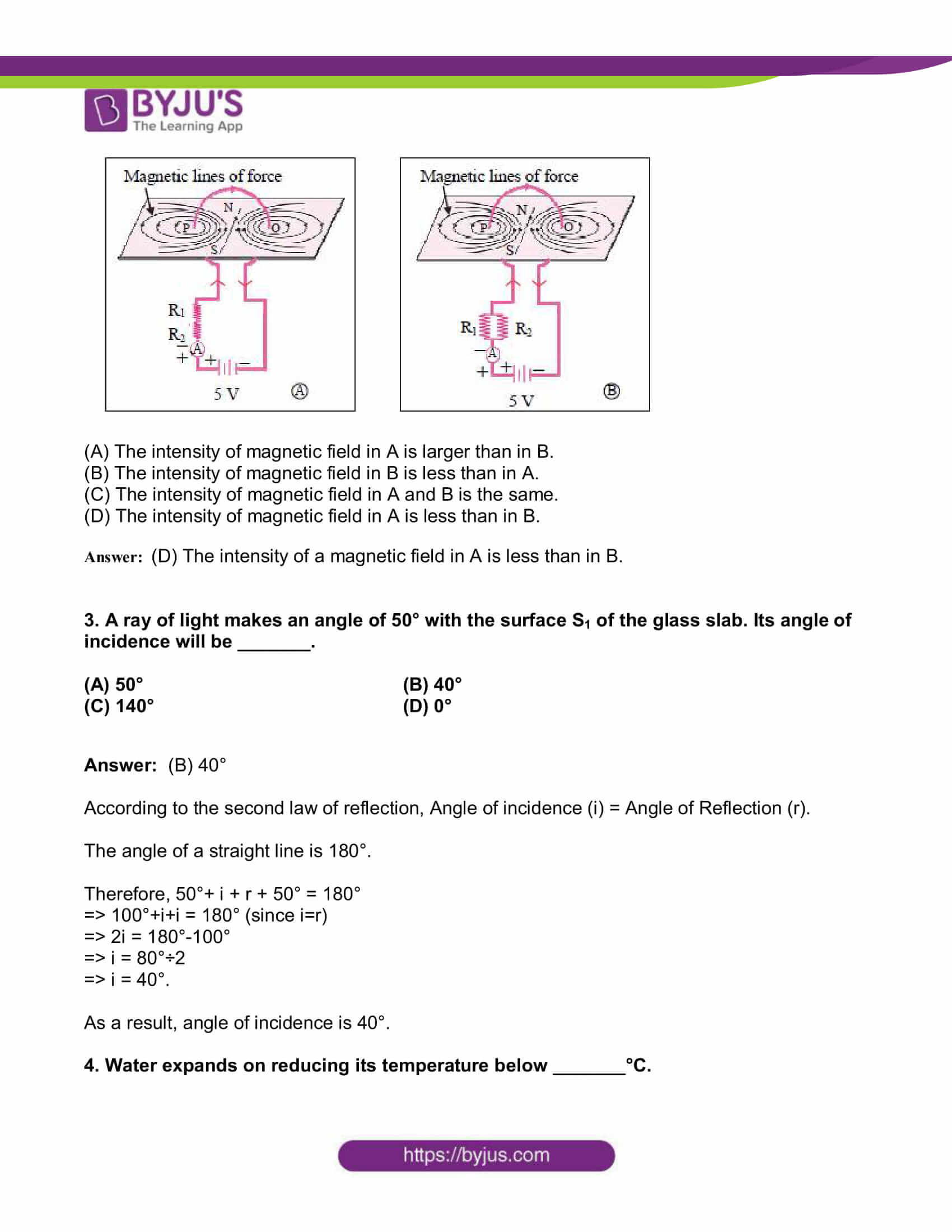
2. Observe the following diagram and choose the correct alternative:
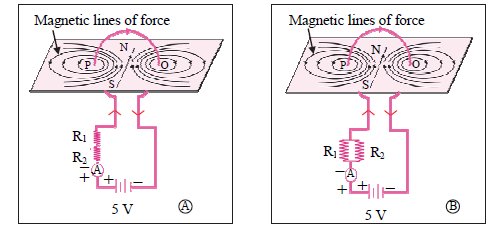
(A) The intensity of magnetic field in A is larger than in B.
(B) The intensity of magnetic field in B is less than in A.
(C) The intensity of magnetic field in A and B is the same.
(D) The intensity of magnetic field in A is less than in B.
Answer: (D) The intensity of a magnetic field in A is less than in B.
3. A ray of light makes an angle of 50° with the surface S1 of the glass slab. Its angle of incidence will be _______.
(A) 50°
(B) 40°
(C) 140°
(D) 0°
Answer: (B) 40°
According to the second law of reflection, Angle of incidence (i) = Angle of Reflection (r).
The angle of a straight line is 180°.
Therefore, 50°+ i + r + 50° = 180°
=> 100°+i+i = 180° (since i=r)
=> 2i = 180°-100°
=> i = 80°÷2
=> i = 40°.
As a result, angle of incidence is 40°.
4. Water expands on reducing its temperature below _______°C.
(A) 0
(B) 4
(C) 8
(D) 12
Answer: (B) 4°C
4°C is the temperature (T) at which liquid water has a minimum volume, at atmospheric pressure. The expansion of water at lower T results from the water molecules arranging themselves to minimize the energy of their interactions.
5. The carbon compound used in daily life is _______.
(A) Edible oil
(B) Salt
(C) Carbon dioxide
(D) Baking soda
Answer: (A) Edible oil
Edible oil is a carbon compound containing unsaturated hydrocarbons. Even if Baking soda and Carbon dioxide also are carbon compounds, they are not as commonly used as edible oils. Hence, the answer is edible oils.
2. Attempt any five of the following questions: [10]
1. Two tungsten bulbs of power 50 W and 60 W work on 220 V potential difference. If they are connected in parallel, how much current will flow in the main conductor?
Answer: 0.5 A
If P1= 50 W
P2= 60 W and
V=220 V, then
To Find I=?
Formula P=VI
Solution =Total Power (P) = P1 + P2 = 50+60 = 110W
So, if P=VI
I= P/V= 110/220= 0.5 A.
2. Give scientific reason:
In the electric equipment producing heat e.g. iron, electric heater, boiler, toaster etc., an alloy such as Nichrome is used, not pure metals.
Answer: An Alloy such as Nichrome has a higher level of resistivity, so they will get heated easily on the passage of even a small amount of current. Also, electric equipment like iron, electric heater, boiler, toaster and so on work based on the heating effect of electric current.
For this reason, nichrome is used in electric equipment producing heat, such as iron, electric heater, boiler and toaster.
3. A metal ball of mass 5 kg falls from a height of 490 m. How much time will it take to reach the ground? (g = 9.8 m/s2).
Answer: 10 Seconds
u (Initial velocity of the metal ball) = 0
s (displacement travelled by the metal ball) =490m
We know that s= ut + 1/2at2
Replacing the values you get 490= 0 x t + ½ x g x t2
(g is acceleration due to gravity)
Hence, 490= 0 x t + ½ x 9.8 x t2
490= 0 + 4.9 x t2= 4.9t2
t2= 490/4.9= 100
So, t=10.
4. Write names of first four homologous series of alcohols:

Answer: Methanol (CH3OH)
Ethanol (C2H5OH)
Propanol (C3H7OH)
Butanol (C4H9OH)
The general formula for the homologous series of alcohols is
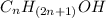 . To get the answer, replace “n” with values.
. To get the answer, replace “n” with values.
n=1
Methanol (CH3OH)
n=2
Ethanol (C2H5OH)
n=3
Propanol (C3H7OH)
n=4
Butanol (C4H9OH).
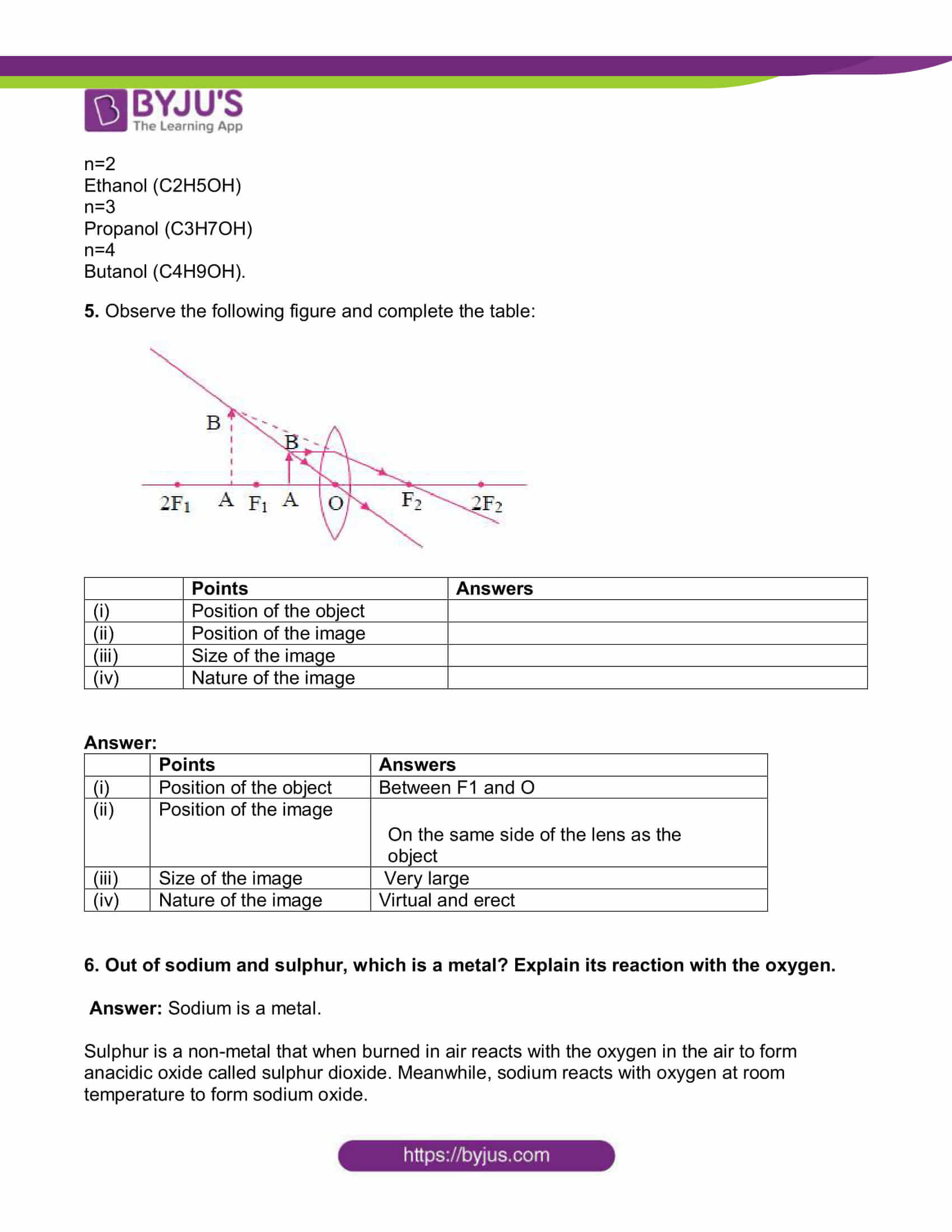
5. Observe the following figure and complete the table:
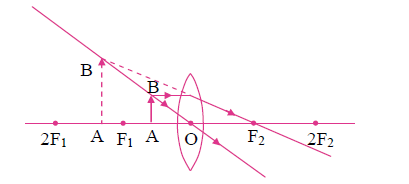
| Points | Answers | |
| (i) | Position of the object | |
| (ii) | Position of the image | |
| (iii) | Size of the image | |
| (iv) | Nature of the image |
Answer:
| Points | Answers | |
| (i) | Position of the object | Between F1 and O |
| (ii) | Position of the image | On the same side of the lens as the object |
| (iii) | Size of the image | Very large |
| (iv) | Nature of the image | Virtual and erect |
6. Out of sodium and sulphur, which is a metal? Explain its reaction with the oxygen.
Answer: Sodium is a metal.
Sulphur is a non-metal that when burned in air reacts with the oxygen in the air to form anacidic oxide called sulphur dioxide. Meanwhile, sodium reacts with oxygen at room temperature to form sodium oxide.
4Na + O2 ————🡪 2Na20
Sodium + Oxygen——🡪Sodium Oxide.
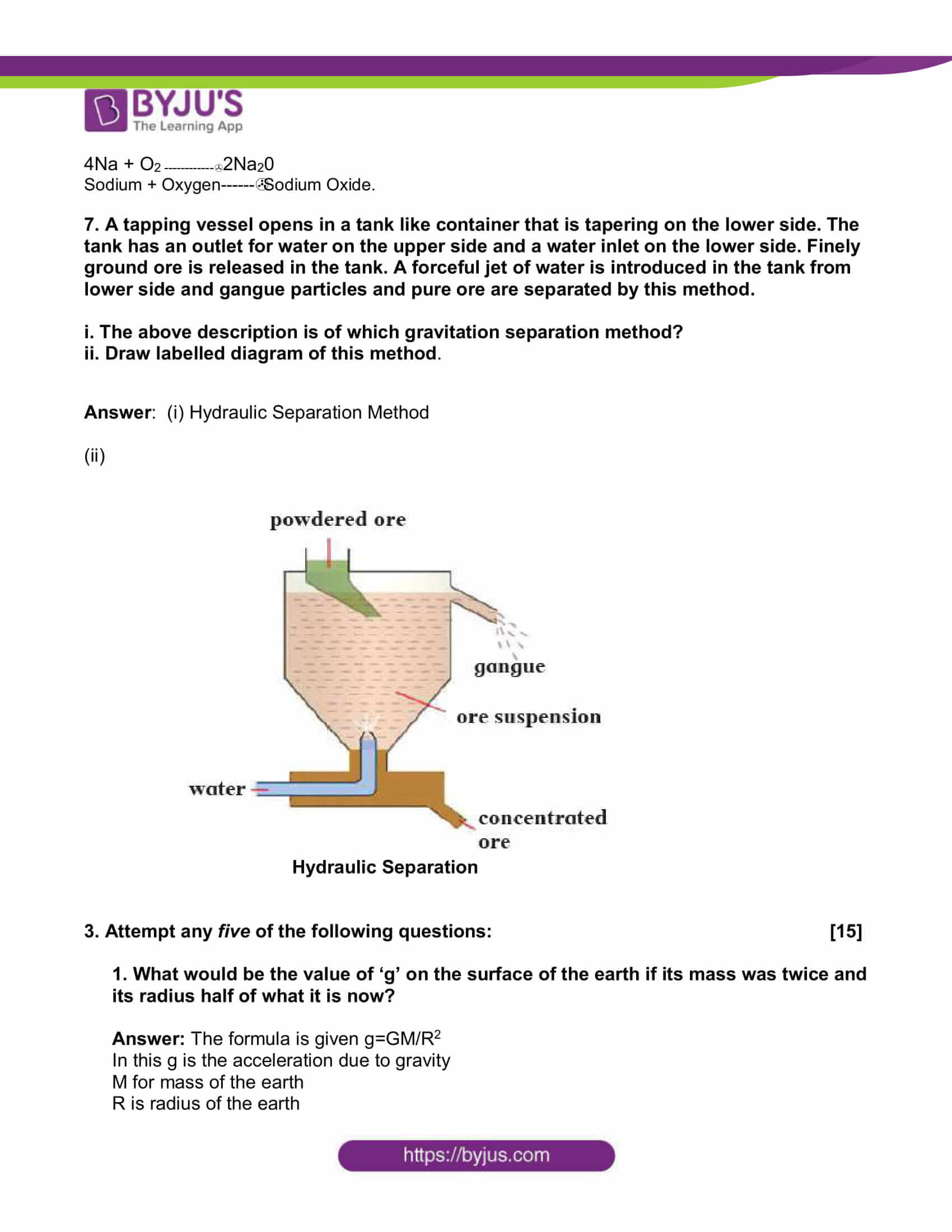
7. A tapping vessel opens in a tank like container that is tapering on the lower side. The tank has an outlet for water on the upper side and a water inlet on the lower side. Finely ground ore is released in the tank. A forceful jet of water is introduced in the tank from lower side and gangue particles and pure ore are separated by this method.
i. The above description is of which gravitation separation method?
ii. Draw labelled diagram of this method.
Answer: (i) Hydraulic Separation Method
(ii)

Hydraulic Separation
3. Attempt any five of the following questions: [15]
1. What would be the value of ‘g’ on the surface of the earth if its mass was twice and its radius half of what it is now?
Answer: The formula is given g=GM/R2
In this g is the acceleration due to gravity
M for mass of the earth
R is radius of the earth
G for universal gravitational constant
Now, according to the question
Mass is twice= 2M
Radius is half= R/2
g1 is considered as the new gravity.
When we substitute the formula we get
g1= Gx2M/(R/2)²
g1= 2GM / R2/4
= 8(GM/ R2)
= 8 x g = 8 (9.8) =78.4 m/s2.
2. Write merits of Mendeleev’s periodic table
Answer: Mendeleev’s periodic table demonstrates the following merits:
1. Mendeleev classified the 63 elements known at the time.
2. Atomic masses of some elements were revised, so as to give them proper place in the
periodic table, in accordance with their properties.
3. Mendeleev kept vacant places in the periodic table for elements not discovered till
then. Three of these unknown elements were given the names eka-boron, eka-aluminium
and eka-silicon from the known neighbours and their atomic masses were indicated as
44, 68 and 72, respectively. Their properties were also predicted.
4. Even though there was no place reserved for noble gases in Mendeleev’s original periodic
table, when noble gases such as helium, neon and argon were discovered towards the end of
19th century, Mendeleev created the ‘zero’ group without disturbing the original periodic table in which the noble gases were fitted very well.
3. Study the following chemical reaction and answer the questions given below:
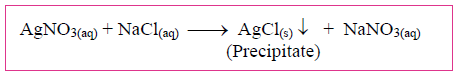
i. Identify and write the type of chemical reaction.
ii. Write the definition of the above type of chemical reaction.
iii. Write the names of reactants and products of the above reaction.
Answer: (i) It is a double replacement chemical reaction.
(ii) The reaction normally takes place in aqueous solutions and the ions in the reactants are exchanged to form a precipitate. These types of reactions are called double displacement reactions.
(iii) Silver Nitrate and Sodium Chloride are the reactants or products used in the above reaction.
4. Explain the following temperature vs. time graph:
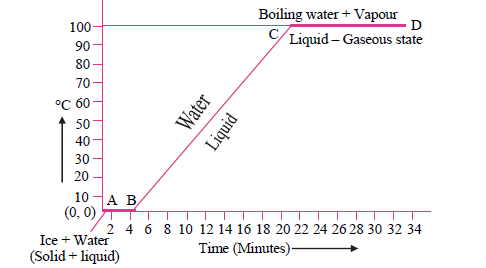
Answer: See the graph given, which represents the changes occurring when a mixture of ice and water is heated. In this graph, the line AB signifies the conversion of ice into the water at a constant temperature. Heated ice melts at 0°C and converts to water even as it maintains the constant temperature at 0°C. This constant temperature is also known as the melting point of ice. Meanwhile, line BC represents the temperature rising from 0°C to 100°C, on further heating. At 100°C water converts to steam, and this is called a boiling point of water. After that, even on heating, the temperature of the water does not rise. Line CD represents this state when there is no change in temperature.
5. Surabhi from Std. X uses spectacles. The power of the lenses in her spectacles is 0.5 D.
Answer the following questions from the given information:
i. Identify the type of lenses used in her spectacles.
ii. Identify the defect of vision Surabhi is suffering from.
iii. Find the focal length of the lenses used in her spectacles.
Answer: (i) Since the power is positive, the lenses used in Surabhi’s spectacle is convex lens
(ii) Surabhi is suffering from hypermetropia. Also, known as long-sightedness, it is a common eye condition, where nearby objects appear blurred. However, your vision is clearer when looking at things further away.
(iii) Power of the lens (P) =1/Focal length (F)
Given that Power = 0.5D
0.5=1/Focal length
Focal length= 1/0.5 =10/5
Hence, Focal length is 2m.
6. Complete the following table:
| Sr. No. | Common Name | Structural Formula | IUPAC Name |
| 1. | Ethylene | CH2=CH2 | ———– |
| 2. | ———— | CH3COOH | Ethanoic Acid |
| 3. | Methyl alcohol | ————- | Methanol |
Answer:
| Sr. No. | Common Name | Structural Formula | IUPAC Name |
| 1. | Ethylene | CH2=CH2 | Ethene |
| 2. | Acetic Acid | CH3COOH | Ethanoic Acid |
| 3. | Methyl alcohol | CH30H | Methanol |
7. What is meant by space debris? Why is there a need to manage the debris?
Answer: In addition to the artificial satellite, there are some other objects revolving around the
earth. These objects include, non-functional satellites, parts of the launcher detached during launching and debris generated due to collision of satellite with other satellites or any other object in the Space. Meanwhile, as per an estimation made in 2016, there are about 2 crore pieces of length more than 1 cm, revolving around the earth. All these are nothing but the debris in space. This debris can be harmful to the artificial satellites. It can collide with these satellites or space crafts and damage them. This debris is increasing day by day. Soon, it will be difficult to launch new space crafts. It is, therefore, very essential to manage the debris.
Q.4. Answer any one of the following questions: (5)
1. Taking into consideration the period of the elements given below, answer the following questions:
| Elements | Atomic Radius (pm) |
| O | 66 |
| B | 88 |
| C | 77 |
| N | 74 |
| Be | 111 |
| Li | 152 |
i. Arrange the above elements in a decreasing order of their atomic radii.
ii. State the period to which the above elements belong.
iii. Why is this arrangement of elements similar to the above period of modern periodic
table?
iv. Which of the above elements have the biggest and the smallest atom?
v. What is the periodic trend observed in the variation of atomic radius while going from
left to right, within a period?
Answer: (i) According to the decreasing order of atomic radii:
Li > Be > B > C > N > O.
(ii) The given elements belong to Period 2.
(iii) You will find that the atomic radius goes on decreasing while going from left to right, within a period. The reason behind this is as follows. While going from left to right, within a period, the atomic number increases one by one, meaning the positive charge on the nucleus increases by one unit at a time. However, the additional electron gets added to the same outermost shell. Due to the increased nuclear charge the electrons are pulled towards the nucleus to a greater extent and thereby the size of the atom decreases.
(iv) In these elements, Lithium has the biggest atom and Oxygen has the smallest atom.
(v) You will find that the atomic radius goes on decreasing while going from left to right within a period.
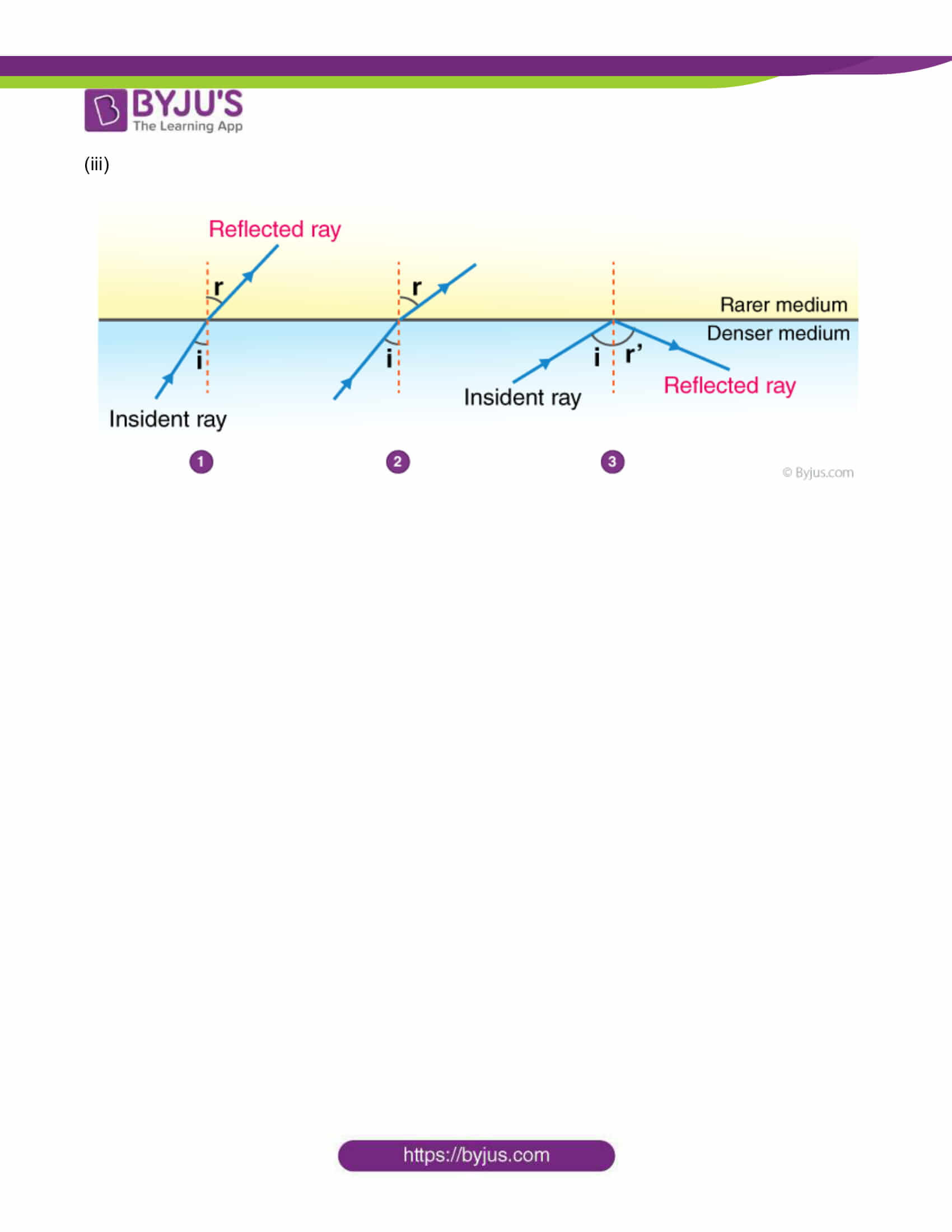
2. The observations made by Swarali while doing the experiment are given below. Based on these, write answers to the questions:
Swarali found that the light ray travelling from the denser medium to rarer medium goes away from the normal. If the angle of incidence (i) is raised by Swarali, the angle of refraction (r) went on increasing. However, after certain value of the angle of incidence the light ray is seen to return into the denser medium.
Questions:
i. What is the specific value of i called?
ii. What is the process of reflection of incident ray into denser medium called?
iii. Draw the diagrams of three observations made by Swarali.
Answers: (i) The specific value of ∠i is called critical angle.
(ii) The process of reflection of incident ray into denser medium is known as total internal reflection.
(iii)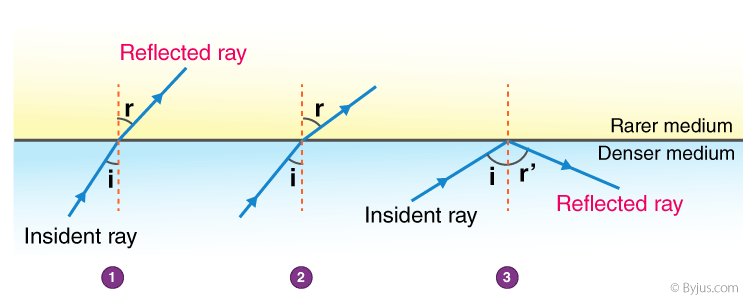
MSBSHSE Class 10 Science Part 2 Questions with Solutions
1. (A) Solve the following questions. [5]
i. Identify the process shown in figure and name it. (1)
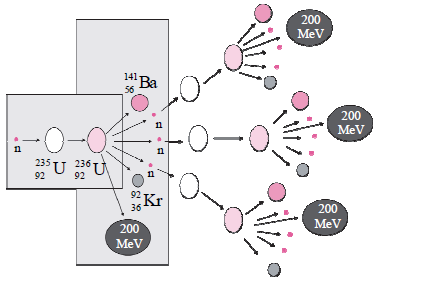
Answer: This process is known as Nuclear Fission (Chain Reaction)
ii. Pranav and Pritee are twins in your class. They belong to _______ twins type. (1)
Answer: dizygotic
Occasionally, two oocytes are released from the ovary of a woman and both oocytes are fertilized by two separate sperms and thus two zygotes are formed. Two embryos are formed from those two zygotes and both of those embryos are separately implanted in the uterus and thus, dizygotic twins are delivered after complete development. Such twins are genetically different and may be of the same or different by gender.
iii. There is an oil layer on the water surface of the river in your area. What will you do? (1)
Answer: Hydrocarbonoclastic bacteria (HCB) like Pseudomonas spp. and Alcanovorax borkumensis are used to clear the oil spills, as they have the ability to destroy the pyridines and other chemicals.
iv. Fill in the boxes with the help of the given clue: (1)
Continuous consumption of alcohol and tobacco material ________
| A | 0 |
Answer:
| A | D | D | I | C | T | I | 0 | N |
v. Find out the correlation: (1)
White revolution: Increase in dairy production: Green revolution: ________.
Answer: Increased production of food grains or crop yield
Various methods applied for harvesting maximum yield from minimum land are collectively called the green revolution.
(B) Choose the correct alternative and rewrite the statements: [5]
i. Somatic and stem cells undergo ________ type of division.
(A) Meiosis
(B) Mitosis
(C) Budding
(D) Cloning
Answer: (B) Mitosis
There are two types of cell division as mitosis and meiosis. Mitosis occurs in somatic
cells and stem cells of the body, whereas meiosis occurs in germ cells.
ii. Which of the following is a man-made disaster?
(A) Earthquake
(B) Flood
(C) Meteoric
(D) Leakage of toxic gases
Answer: (D) Leakage of toxic gases
Earthquake, Flood and Meteoric are all natural disasters, while leakage of toxic gases is man-made.
iii. In a food chain, autotrophic plants are present at the _______ level.
(A) Tertiary nutrition
(B) Secondary nutrition
(C) Producer
(D) Apex
Answer: (C) Producer
Autotrophic plants are present at the producer level of the food chain.
iv. ________ is a cold blooded animal.
(A) Bat
(B) Snake
(C) Rabbit
(D) Elephant
Answer: (B) Snake
Reptiles are considered to be cold-blooded animals as they have the ability to maintain their body temperature according to changes in an environment. Snakes are reptiles and hence, are considered to be cold-blooded animals.
v. ________ is a connecting link between annelida and arthropoda.
(A) Duck-billed platypus
(B) Peripatus
(C) Lungfish
(D) Whale
Answer: (B) Peripatus
Some plants and animals show some morphological characters by which they can be
related to two different groups; hence, they are called ‘connecting links’. Ex. In Peripatus,
characters like segmented body, thin cuticle, and parapodia-like organs are present.
Similarly, these animals show tracheal respiration and open circulatory system similar to
arthropods. This indicates that Peripatus is connecting link between annelida and arthropoda.
2. Solve any five of the following questions: [10]
i. Complete the following chart:
Answers:
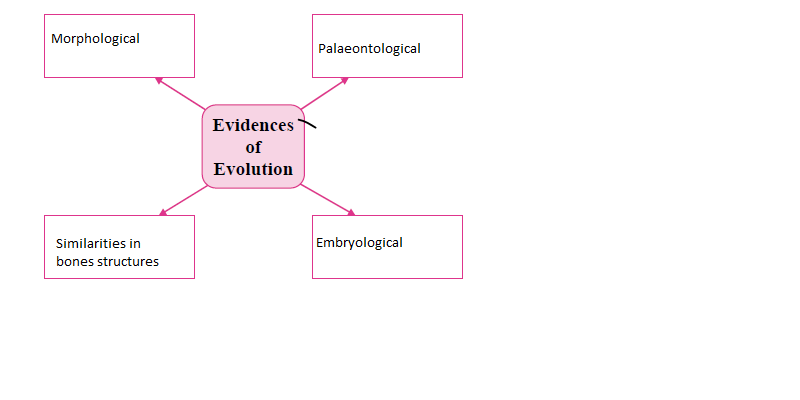
ii. Draw a neat diagram of the structure of chromosome and label the parts:
A. Centromere
B. p-arm.
Answer:

iii. What are the advantages of hydroelectric power generation?
Answer: Given below are the benefits of hydroelectric power generation:
- It is a clean source of energy. Since no fuel is burnt in hydroelectric plants, it results in no air pollution due to combustion of fuel results.
- Since water is used in the generation of energy, if there is sufficient water storage in the Dam, it is possible to generate electricity, as and when necessary.
- Although water reservoir is used for power generation, it can be replenished during the rainy season, leading to uninterrupted power generation.
iv. State any four benefits of animal classification.
Answer: Benefits of animal classification are as given below:
1. The study of animals is made more convenient with animal classification.
2. The study of a few animals from a group helps to understand the entire animal group.
3. Animal classification gives an idea about animal evolution.
4. Animals can be easily identified with great accuracy.
5. It helps to understand the relationship of animals with other living organisms.
6. It helps to understand the habitat of each animal and its exact role in nature.
7. It helps to understand various adaptations shown by animals.
v. State any four objectives of disaster management.
Answer: Given below are the objectives of disaster management:
1. Save human life by evicting the people away from the place of disaster
2. Reduce the effect of disaster by supplying essential commodities to the people
3. To restore human life in the disaster region by creating reconciliation in disaster.
4. Rehabilitate the disaster victims.
5. Considering protective or preventive measures in disaster, so as to reduce the intensity of future disasters.
vi. Give scientific reason:
Microbial enzymes are used instead of chemical catalyst in a chemical industry.
Answer: Instead of chemical catalysts, microbial enzymes are used in the chemical industry. These enzymes are active at low temperature, pH and pressure; due to which energy is saved and erosion-proof instruments are also not necessary. Enzymes carry out specific processes; hence, unnecessary by-products are not formed due to which expenses on purification are minimised. In case of microbial enzymatic reactions, elimination and decomposition of waste material is avoided and enzymes can be reused. Hence, such enzymes are eco-friendly.
vii. Read the following extract and answer the questions that follow:
A liberal view behind the concept of organ and body donation is that after death our body should be useful to other needy persons so that their miserable life would become comfortable. Awareness about these concepts is increasing in our country and people are voluntarily donating their bodies.
Lives of many people can be saved by organ and body donation. Blinds can regain their vision. Life of many people can be rendered comfortable by donation of organs like liver, kidneys, heart, heart valves, skin etc. Similarly, the body can be made available for research in medical studies. Many government and social organizations are working towards increasing the awareness about body donation.
a. What is the liberal view behind organ and body donation?
b. Name any four organs that can be donated.
Answer: a. A liberal view behind the concept of organ and body donation is that after death our body should be useful to other needy persons so that their miserable life would become comfortable.
b. Four organs that can be donated are organs like liver, kidneys, heart, heart valves, skin etc.
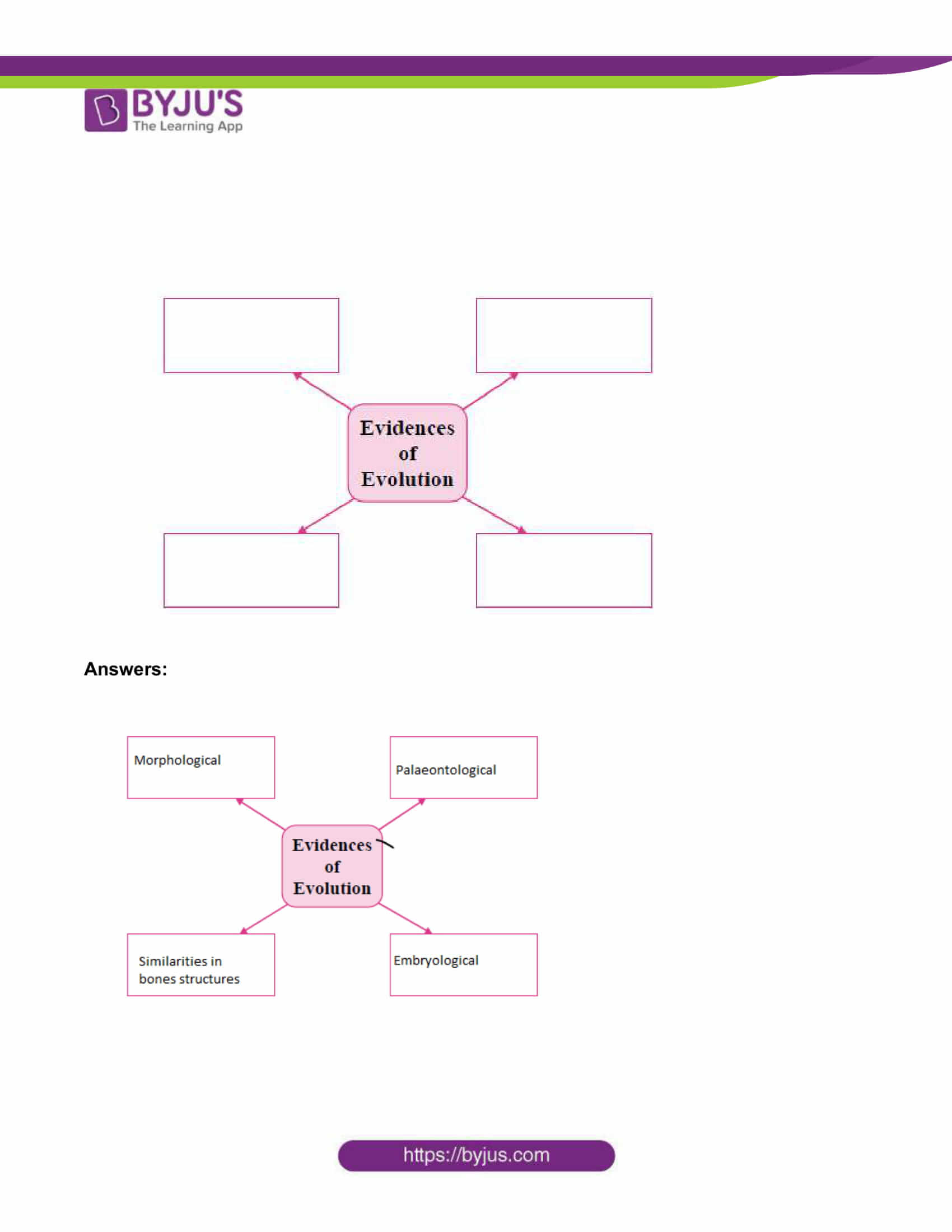
3. Solve the following questions (any five): [15]
i. Answer the following questions:
a. What do you mean by central dogma?
b. What is transcription?
c. What is meant by triplet codon?
Answers: a. Process of synthesizing proteins by DNA through the RNA is called “Central Dogma.”
b. mRNA is produced as per the sequence of nucleotides on DNA. Only one of the two strands of DNA is used in this process. The sequence of nucleotides in mRNA being produced is always complementary to the DNA strand used for synthesis. Besides, there is uracil in RNA instead of thymine of DNA. This process of RNA synthesis is called ‘transcription’.
C. The mRNA formed in nucleus comes in cytoplasm bringing in the coded message from DNA containing the codes for amino acids. The code for each amino acid consists of three nucleotides called ‘triplet codon’.
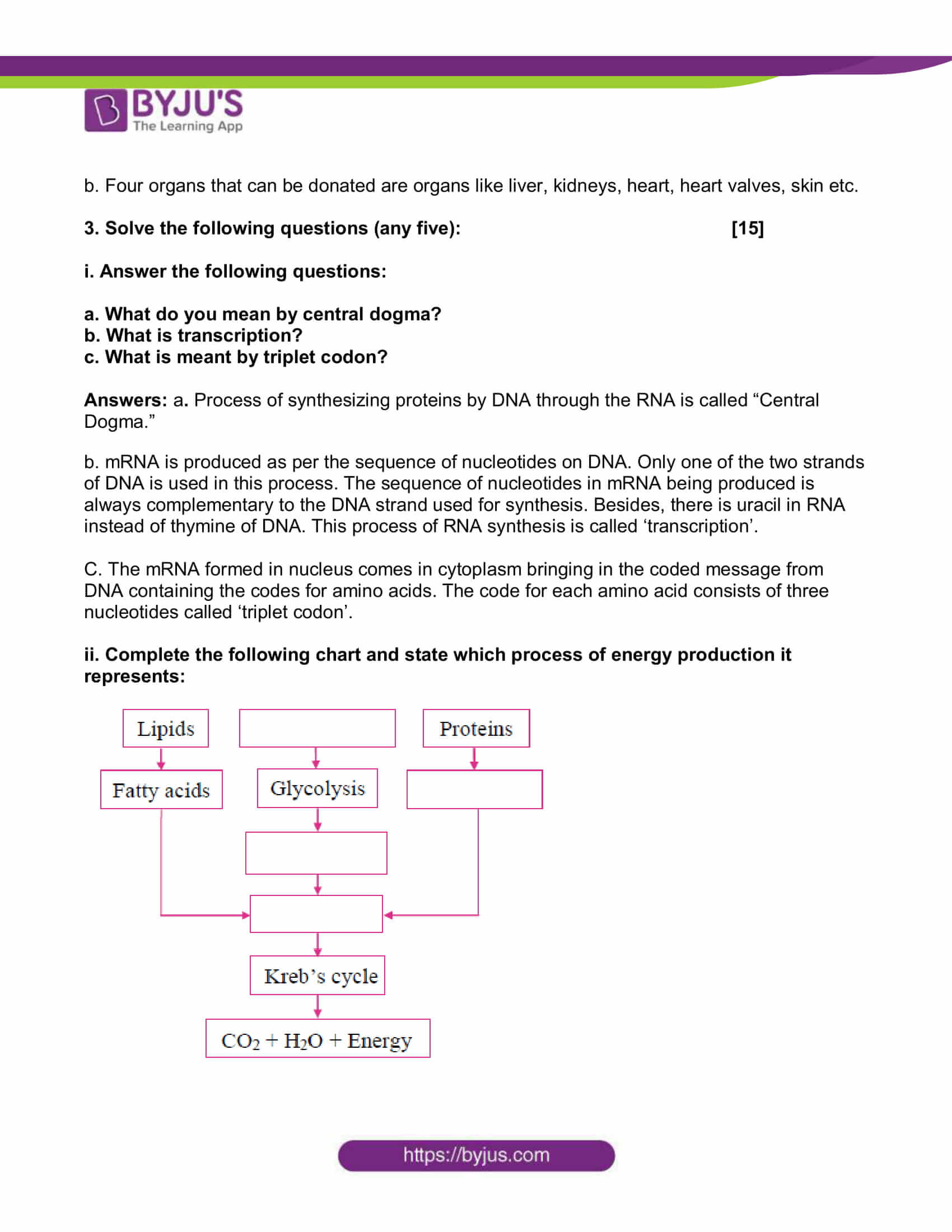
ii. Complete the following chart and state which process of energy production it represents:
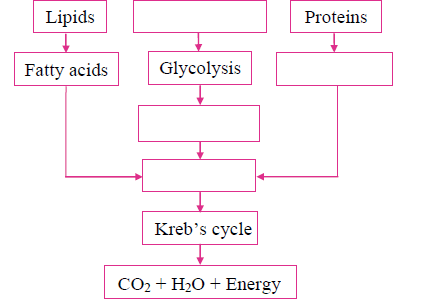
Answer:
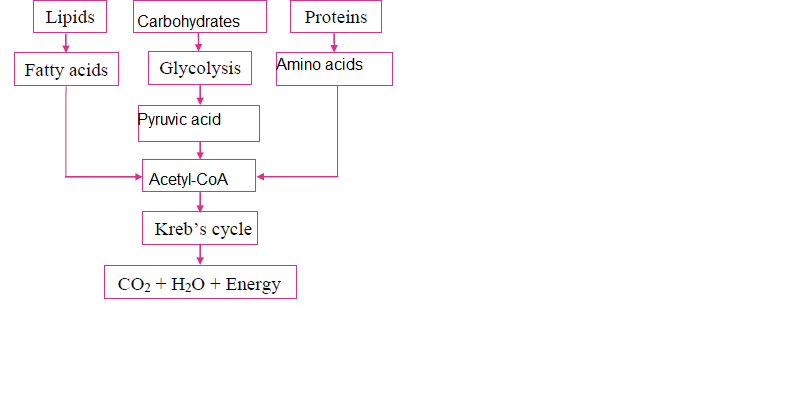
iii. a. Playing games on mobile while eating is right or wrong. Justify.

Answer: It is wrong. If you play games on mobile while eating your entire concentration will be on the game and so will not digest the food properly. Hence, you should respect food and concentrate on that while eating, instead of on your mobile.
b. What do you conclude from the following picture?

Answer: The image is a message about addiction control. You have to control and stay away from addictions such as smoking, drugs, alcohol and so on.
C. Observe the following picture and state what can be the outcome?
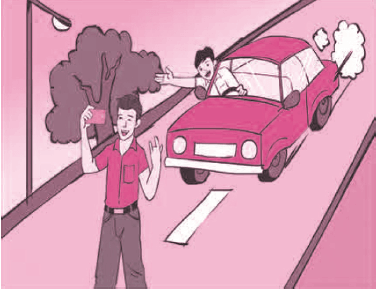
Answer: Taking a selfie on the road could lead to an accident, as concentration would be on taking the photo and not on the vehicles on the road.
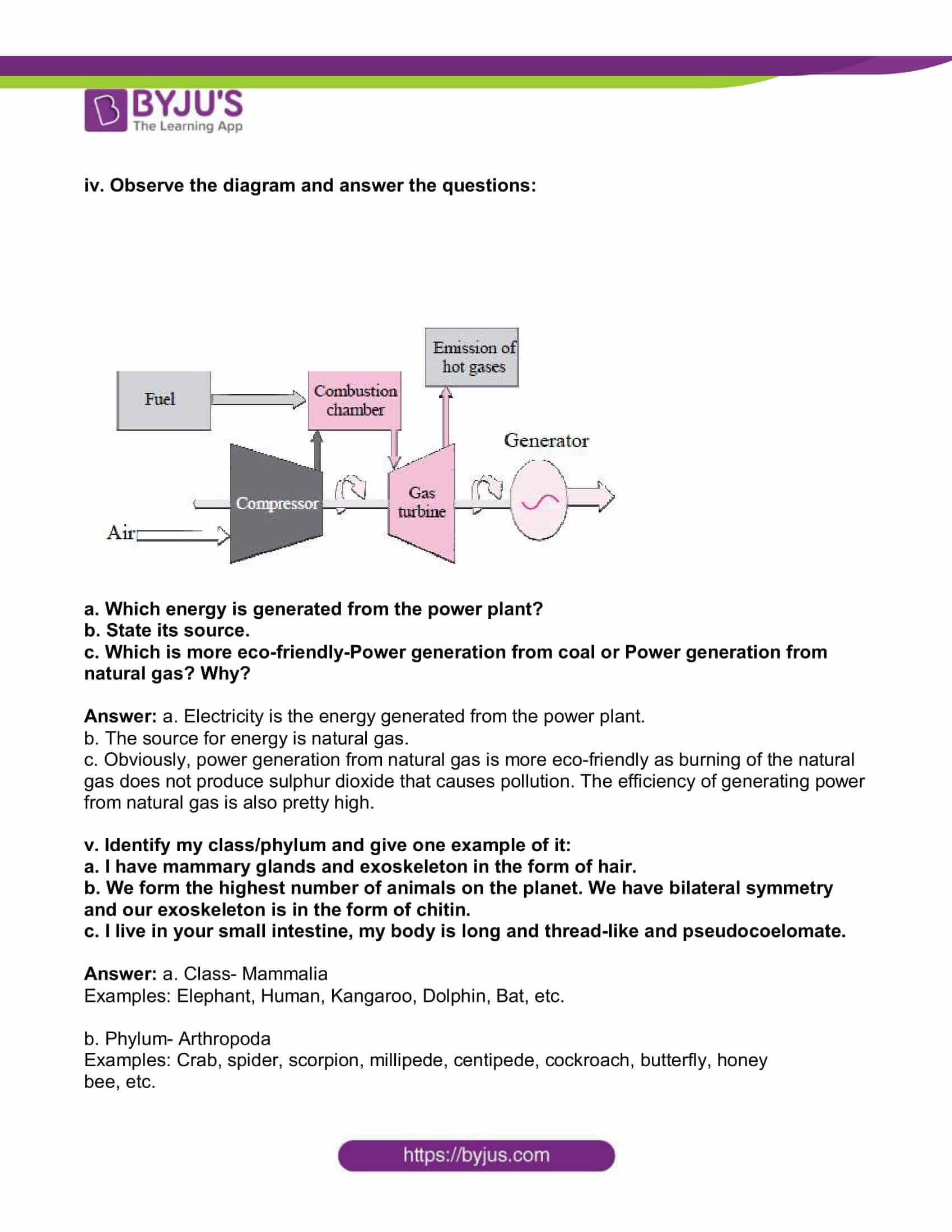
iv. Observe the diagram and answer the questions:
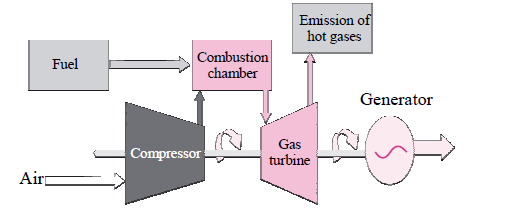
a. Which energy is generated from the power plant?
b. State its source.
c. Which is more eco-friendly-Power generation from coal or Power generation from
natural gas? Why?
Answer: a. Electricity is the energy generated from the power plant.
b. The source for energy is natural gas.
c. Obviously, power generation from natural gas is more eco-friendly as burning of the natural gas does not produce sulphur dioxide that causes pollution. The efficiency of generating power from natural gas is also pretty high.
v. Identify my class/phylum and give one example of it:
a. I have mammary glands and exoskeleton in the form of hair.
b. We form the highest number of animals on the planet. We have bilateral symmetry and our exoskeleton is in the form of chitin.
c. I live in your small intestine, my body is long and thread-like and pseudocoelomate.
Answer: a. Class- Mammalia
Examples: Elephant, Human, Kangaroo, Dolphin, Bat, etc.
b. Phylum- Arthropoda
Examples: Crab, spider, scorpion, millipede, centipede, cockroach, butterfly, honey
bee, etc.
c. Phylum- Aschelminthes
Examples: Ascaris (Intestinal worm), Filarial worm, Loa loa (Eye worm), etc.
vi. Observe the following figure and answer the questions:
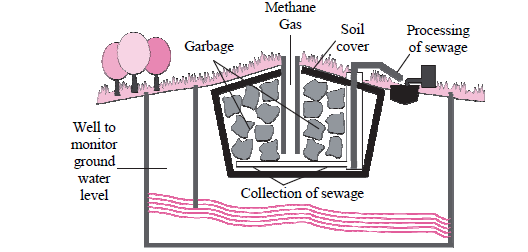
a. Identify the process shown in the figure.
b. Explain the process in short.
Answer: a. The process shown in the figure is known as Modern Landfill site.
b. Degradable waste collected in urban areas is used for this purpose. Large pits are dug in open spaces far away from the residential area and those pits are lined with plastic sheets as a precaution against pollution of soil, due to leaching of toxic and harmful materials. Compressed waste is dumped in the pit. It is covered with layers of soil, saw dust, leafy waste and specific biochemical. Bioreactors are mixed at some places. Microbes present in soil and other top layers decompose the waste. Completely filled pit is sealed with soil slurry. Best quality compost is formed after a few days. Such land filling sites can be reused after removal of compost.
vii. a. What is biotechnology?
b. Explain any two commercial applications of it.
Answer: a. Biotechnology is a field of science that brings about artificial genetic changes and hybridization in organisms for human welfare. Various branches of Science like Cytology, Biochemistry, Molecular Biology, and Genetic Engineering are included in Biotechnology.
b. Two Commercial Applications of Biotechnology are given below:
Crop Biotechnology: Biotechnology is used in the agricultural field to improve yield and variety. Some of the areas where loads of research is being conducted includes Hybrid Seeds, Genetically Modified Crops, Herbicide tolerant plants, Bio fertilizers and so on.
Human Health: Diagnosis and treatment of the diseases are two important aspects of human health management. Biotechnology helps to identify the role of gene, if any, in the disease of a person. Diagnosis of diabetes and heart diseases has become possible even before the onset of symptoms, with the help of biotechnology. Diagnosis of diseases like AIDS, dengue can be done within a few minutes. Hence, treatment can be done at the earliest.
4. Solve any one of the following questions: [5]
i. a. identify the following symbols and state their significance: (2)

Answer: (1) Save Water.
Significance: Water is essential. Don’t run the tap needlessly and waste water.
(2) Use a bicycle.
Significance: It’s a non-polluting environment friendly vehicle.
b. How can biodiversity be conserved? (3)
Answer: Given below are some of the points on how biodiversity is being conserved:
1. Rare species of organisms were protected.
2. National parks and sanctuaries were established.
3. Some regions were declared ‘bioreserves’.
4. Projects were set up for conservation of special species.
5. All plants and animals were conserved.
6. The rules were observed.
7. A record of traditional knowledge was maintained.
ii. Answer the following questions:
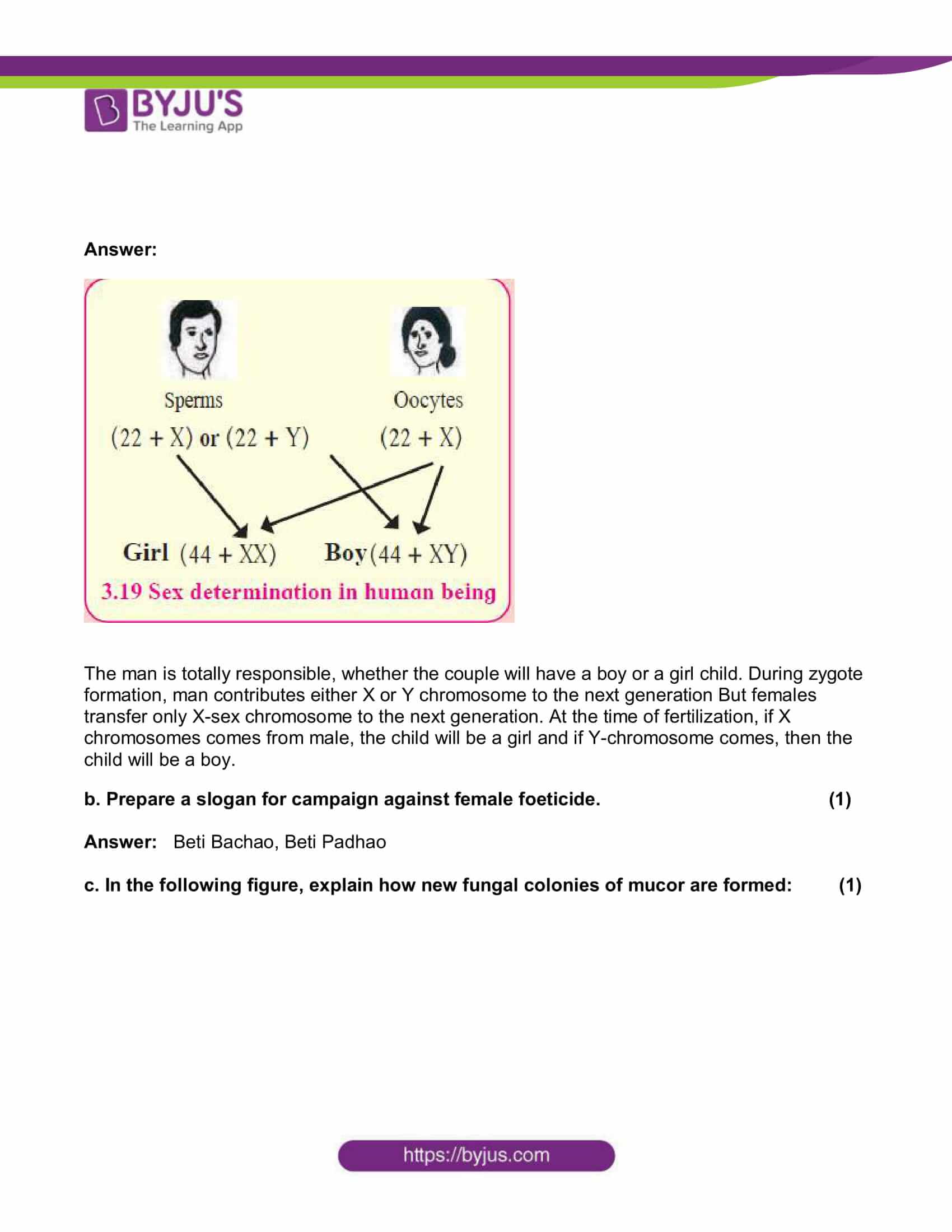
a. “Gender of a child is determined by the male partner of couple.” Draw a diagram explaining the above statement. (2)
Answer:
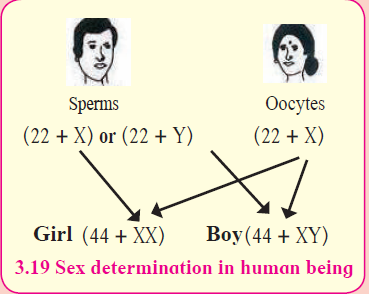
The man is totally responsible, whether the couple will have a boy or a girl child. During zygote formation, man contributes either X or Y chromosome to the next generation But females transfer only X-sex chromosome to the next generation. At the time of fertilization, if X chromosomes comes from male, the child will be a girl and if Y-chromosome comes, then the child will be a boy.
b. Prepare a slogan for campaign against female foeticide. (1)
Answer: Beti Bachao, Beti Padhao
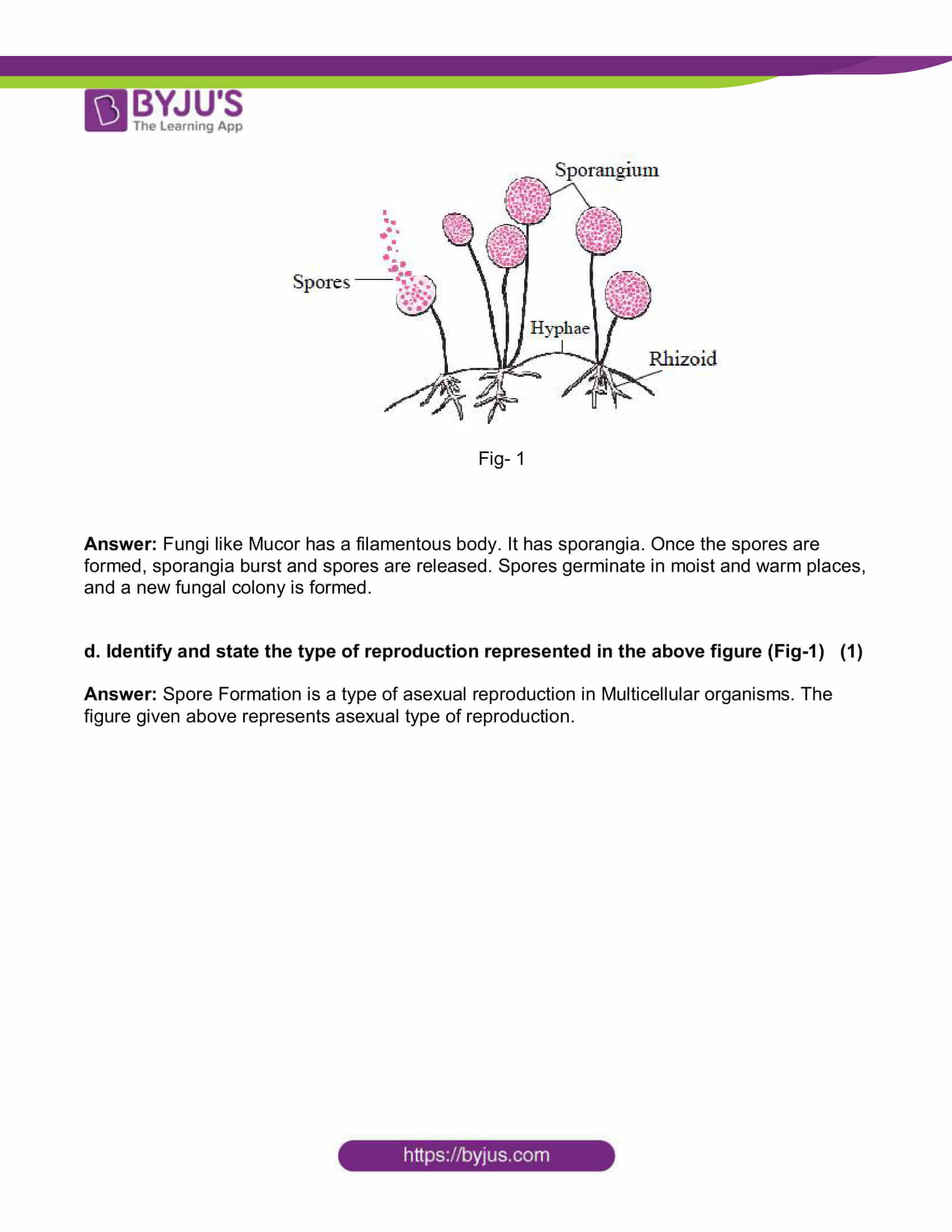
c. In the following figure, explain how new fungal colonies of mucor are formed: (1)
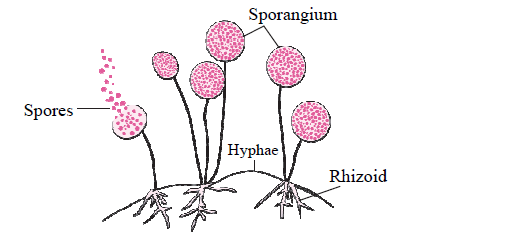
Fig- 1
Answer: Fungi likeMucor has a filamentous body. It has sporangia. Once the spores are formed, sporangia burst and spores are released. Spores germinate in moist and warm places, and a new fungal colony is formed.
d. Identify and state the type of reproduction represented in the above figure (Fig-1) (1)
Answer: Spore Formation is a type of asexual reproduction in Multicellular organisms. The figure given above represents asexual type of reproduction.















Comments