In 1961, Peter Mitchell postulated the Chemiosmotic hypothesis. It explains the mechanism of ATP synthesis within chloroplast during photosynthesis. During the photochemical phase or light reaction, ATP and NADP are generated. These are the key components and used in the dark reaction for the production of the final product of photosynthesis i.e. sugar molecules. Let us see how this ATP and NADPH are generated during the light reaction.
As per the chemiosmotic hypothesis, ATP production is the outcome of the proton gradient established across the membrane of thylakoids. The required components for chemiosmosis are proton gradient, proton pump, and ATP synthase. ATP synthase is an enzyme aiding in bringing about ATP synthesis. The enzyme has two portions -F0 and F1. F0 is a transmembrane channel while configuration changes in F1 activate the enzymes. They phosphorylate ADP. One of the driving factors of ATP Synthase is the protein gradient that is developed across a membrane.
In plants, during the light reaction, photosystems help chlorophyll to absorb light. As a result, hydrolysis (splitting of water) takes place and releases electrons and protons. The electrons get excited to higher energy level and are transported by the electron transport system while protons (hydrogen ions) from the stroma starts to accumulate inside the membrane. This creates a proton gradient. Some protons are used by photosystem I for reduction to NADPH. When the proton gradient is collapsed, it releases energy and protons are carried out back to stroma via F0, the transmembrane channel of ATP synthase. This released energy causes changes in F1 configuration and triggers the ATP synthase to convert ADP to ATP.
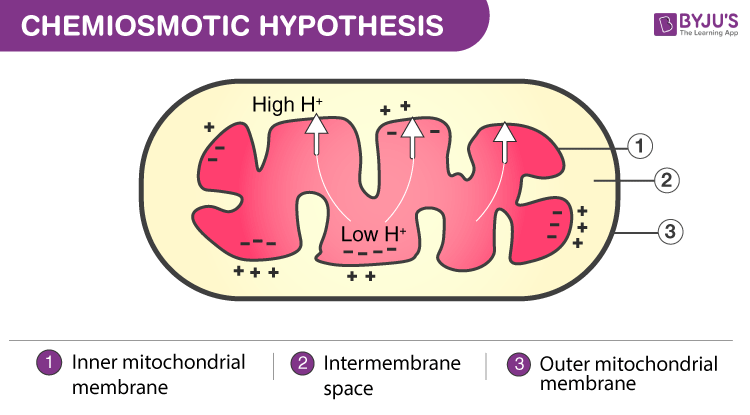
The Chemiosmotic Theory
According to this theory, molecules like glucose are metabolized to develop acetyl CoA in the form of an intermediate that is energy-rich. The proper oxidation of acetyl CoA occurs in the mitochondrial matrix and is combined to the reduced form of a carrier molecule namely FAD and NAD. The carriers then supply electrons to the transport chain of the electron in the inner membrane of mitochondria, which further supply them to different other proteins present in the ETC. The energy present in the electrons is basically used to pump out protons from the matrix in the inner mitochondrial membrane. It is used for energy storage in the form of a transmembrane electrochemical gradient.
The protons return to the inner membrane by the ATP enzyme synthase. The proton-flow travels into the matrix of mitochondria through ATP synthase, which gets a good amount of energy for the ADP to integrate with inorganic phosphate to produce ATP.
For more information regarding the Chemiosmotic Hypothesis, visit BYJU’S.

Comments