TN 10th Standard Science Exam Question Papers 2017 with Solutions – Free Download
TN SSLC Board Class 10 Science 2017 Question Paper with solutions is considered as the best study material for those students who are aiming for high scores in the exams. They can download these questions and practise hard. This Class 10 exam question paper is used by students to score well in the Science paper of the board exams. Referring to the solutions given here after answering the TN Board SSLC Class 10 Science Previous Year Question Paper 2017 PDF will help the students to gauge their performance and plan their studies.
Students can now, with just a click, download the PDF formats of the solved or the unsolved question paper, as their preference. These clickable links are listed below in this article, along with the questions and answers on the web-page. Meanwhile, the students who have mastered all these complex concepts and formulas in the subject will find it easier to score high marks. They will find it easy to ace the TN Board Class 10 Science exam, as the questions could be based on the complex formulas and equations of the subject.
Students are encouraged to revise the entire subject for the exam and to solve the previous papers of TN Board Class 10 Science. Solving these papers is the best way to get an overview of the exam pattern with the marking scheme. Students can also gauge the difficulty level of the exams as well as scale their exam preparations with the help of these question paper solutions.
Download TN Board SSLC 2017 Science Question Paper PDF
Download TN Board SSLC 2017 Science Question Paper With Solutions PDF
PART- III
Section-I 15 X 1= 15
-
- In persons’ suffering from insulin-dependent diabetes,___________ the cells of pancreas are degenerated.
(Alpha, Beta, Gamma, Delta)
Answer: Beta
-
- The first vaccine injected into a just born baby is__________
(Oral Polio, DPT, DPT and Oral Polio, BCG)
Answer: BCG
-
- The endocrine gland related to the immune system is__________
(Thyroid, Thymus, Adrenal, Pineal)
Answer: Thymus
-
- If the water-soaked seed is pressed, a small drop of water comes out through the_________
(Stomata, Lenticle, Micropyle, Radicle)
Answer: Micropyle
-
- Mitral Valve is found between___
(Right auricle and right ventricle, Left auricle and left ventricle, Right ventricle and pulmonary artery, Left ventricle and aorta)
Answer: Left auricle and left ventricle
-
- In Monotropa the special type of root, which absorbs nourishment is_________
(Haustoria, Mycorrhizal root, Clinging root, Adventitious root)
Answer: Mycorrhizal root
-
- The sedimented and floating materials are removed by this treatment process. Which one?
(Primary treatment, Secondary Treatment, Tertiary Treatment, Peripheral Treatment)
Answer: Primary treatment
-
- The mixture of gases used by deep sea divers is______
(helium-oxygen, oxygen-nitrogen, hydrogen-nitrogen)
Answer: helium-oxygen
-
- Vinegar is present in acetic acid. Curd contains_______ acid.
(Lactic acid, Tartaric acid, Citric acid)
Answer: Lactic acid
-
- An element which is an essential constituent of all organic compounds belongs to the ______ group.
(14th group, 15th group, 16th group)
Answer: 14th group
-
- _______ is used for coagulating rubber from latex.
(Ethanol, Ethanoic acid)
Answer: Ethanoic acid
-
- Screw Gauge is an instrument used to measure the dimensions of a very small objects up to ___________
(0.1 cm, 0.01 cm, 0.1 mm, 0.01 mm)
Answer: 0.01 mm
-
- The physical quantity, which is equal to the rate of change of momentum is_______
(displacement, acceleration, force, impulse)
Answer: force
-
- Kilowatt-hour is the unit of_____
(potential difference, electric power, electric energy, charge)
Answer: electric energy
-
- An electric current through a metallic conductor produces_______ around it.
(magnetic field, mechanical force, induced current)
Answer: magnetic field
Section-II 20 X 2= 40
-
- The inheritable characters vary in different species and within the same species. Name the variations in the following cases.
(i) The eye colour among human beings are varied as blue, black, brown, green etc. This is called as __________ variation
(ii) The dentition in the rabbit and the elephant are not the same. This is called as ________ variation.
Answer: (i) intraspecific variation
(ii) interspecific variation
-
- What is Genetic Engineering?
Answer: The manipulation and transfer of genes from one organism to another organism to create a new DNA called the recombinant DNA(rDNA) is called Genetic Engineering. The term recombinant is used because DNA from two different sources can be joined together. Hence, genetic engineering is also called recombinant DNA technology.
-
- Match the following by identifying the pairs
(medicines, fuel, microbes, metabolism, organic acids)
| (i) Vaccine | |
| (ii) Natural gas | |
| (iii) Citric acid | |
| (iv) Vitamins |
Answer:
| (i) Vaccine | Microbes |
| (ii) Natural gas | Fuel |
| (iii) Citric acid | Organic acid |
| (iv) Vitamins | Metabolism |
-
- Marasmus and Kwashiorkor are both protein deficiency defects. Marasmus differs from Kwashiorkor in the enlarged belly and swelling in the face. Are these symptoms for the disease correct? If not, correct it.
Answer: The symptoms of the disease given here are wrong. The symptoms for Kwashiorkor include enlarged belly and swelling of the ankles and feet. For Marasmus, the symptoms are shrunken, wasted appearance, loss of muscle mass and subcutaneous fat mass.
-
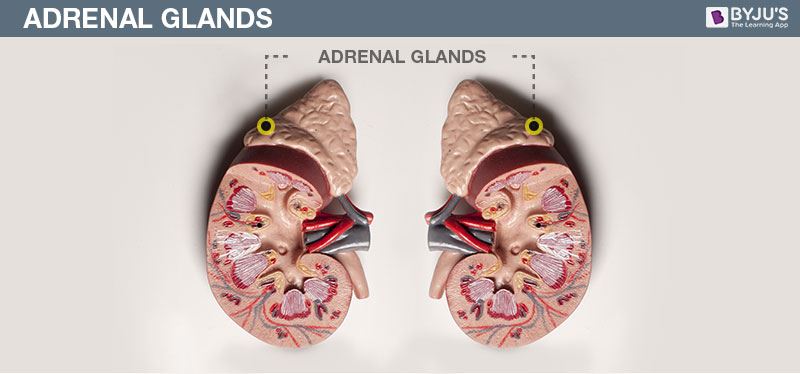
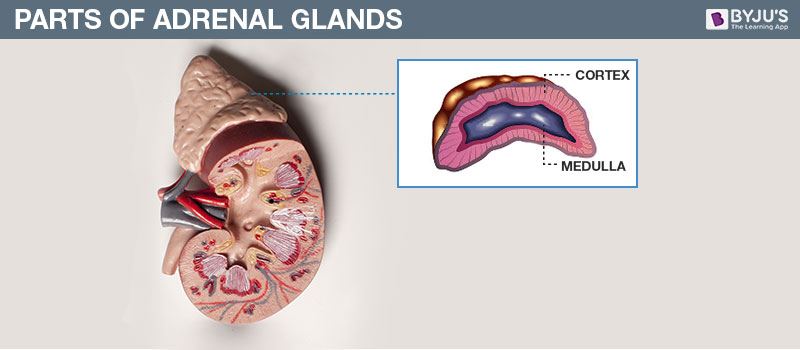
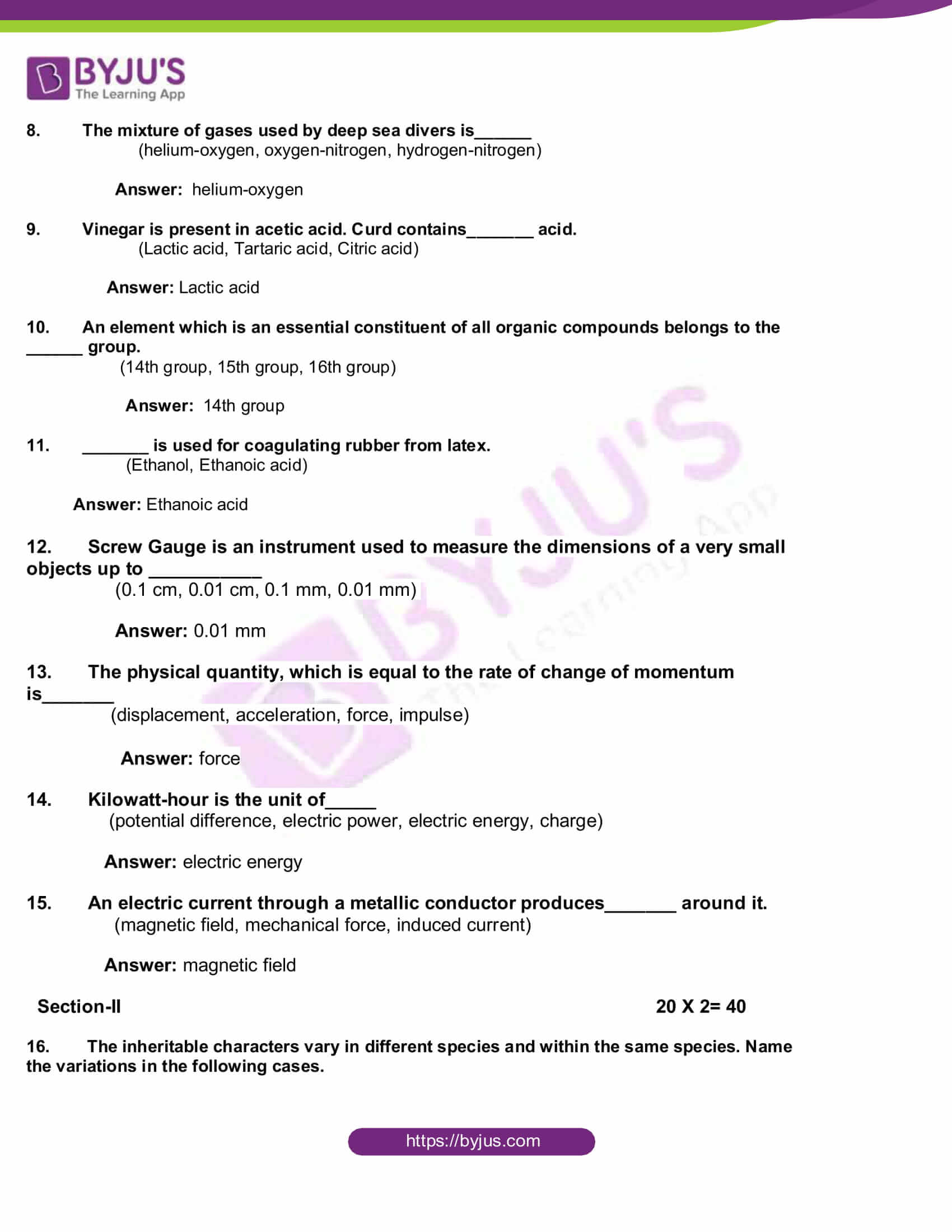
- Copy the diagram and label the parts with the help of the clues given:
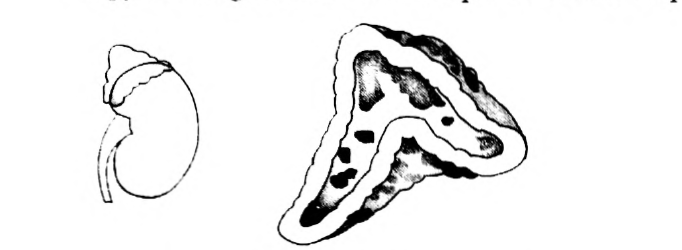
(i) It is otherwise called supra renal gland
(ii) It secretes two hormones, namely aldosterone and cortisone
Answer:
-
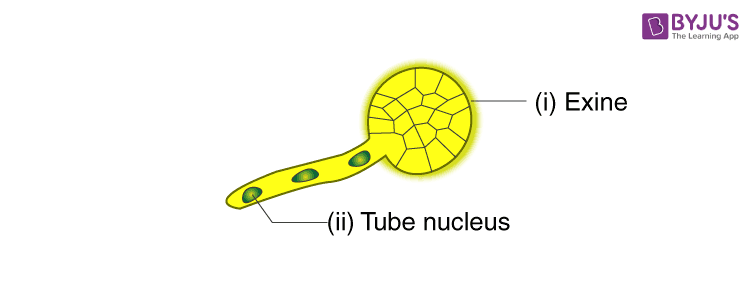
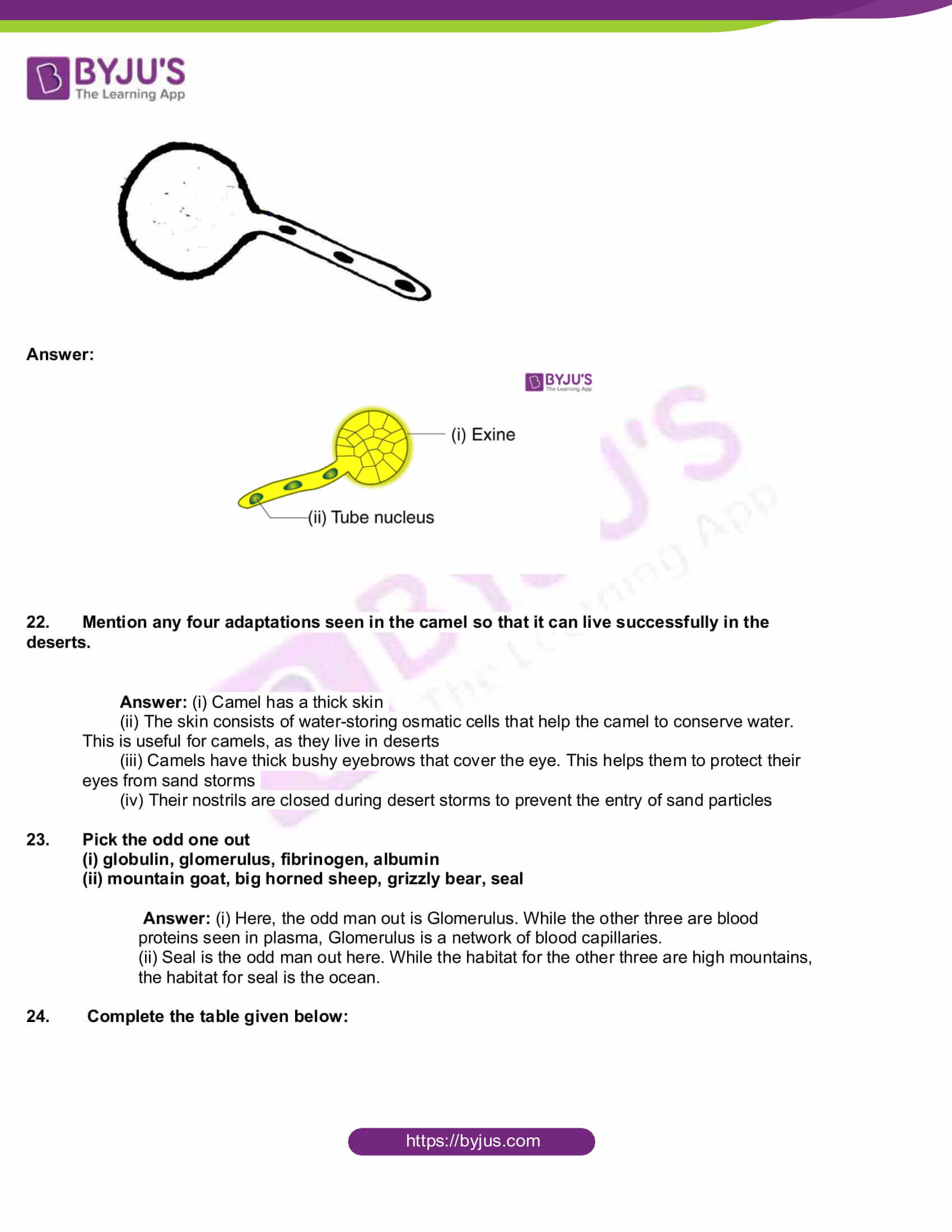
- Draw the given diagram and label the following part:
(i) Exine
(ii) Tube Nucleus
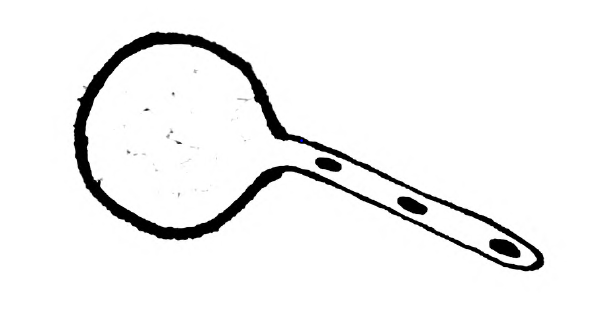
Answer:
-
- Mention any four adaptations seen in the camel so that it can live successfully in the deserts.
Answer: (i) Camel has a thick skin
(ii) The skin consists of water-storing osmatic cells that help the camel to conserve water. This is useful for camels, as they live in deserts
(iii) Camels have thick bushy eyebrows that cover the eye. This helps them to protect their eyes from sand storms
(iv) Their nostrils are closed during desert storms to prevent the entry of sand particles
-
- Pick the odd one out
(i) globulin, glomerulus, fibrinogen, albumin
(ii) mountain goat, big horned sheep, grizzly bear, seal
Answer: (i) Here, the odd man out is Glomerulus. While the other three are blood proteins seen in plasma, Glomerulus is a network of blood capillaries.
(ii) Seal is the odd man out here. While the habitat for the other three are high mountains, the habitat for seal is the ocean.
-
- Complete the table given below:
| Excretory Organ | Disposed as | Excretory Products |
| Kidneys | Urine | Nitrogenous waste products-Urea, Uric acid, Creatinine etc |
| Lungs | Exhaled/Expired Air | __________________________ |
| Skin | _______________ | Excess water and salts |
Answer:
| Excretory Organ | Disposed as | Excretory Products |
| Kidneys | Urine | Nitrogenous waste products-Urea, Uric acid, Creatinine etc |
| Lungs | Exhaled/Expired Air | Water vapour and Carbon Dioxide |
| Skin | Sweat | Excess water and salts |
-
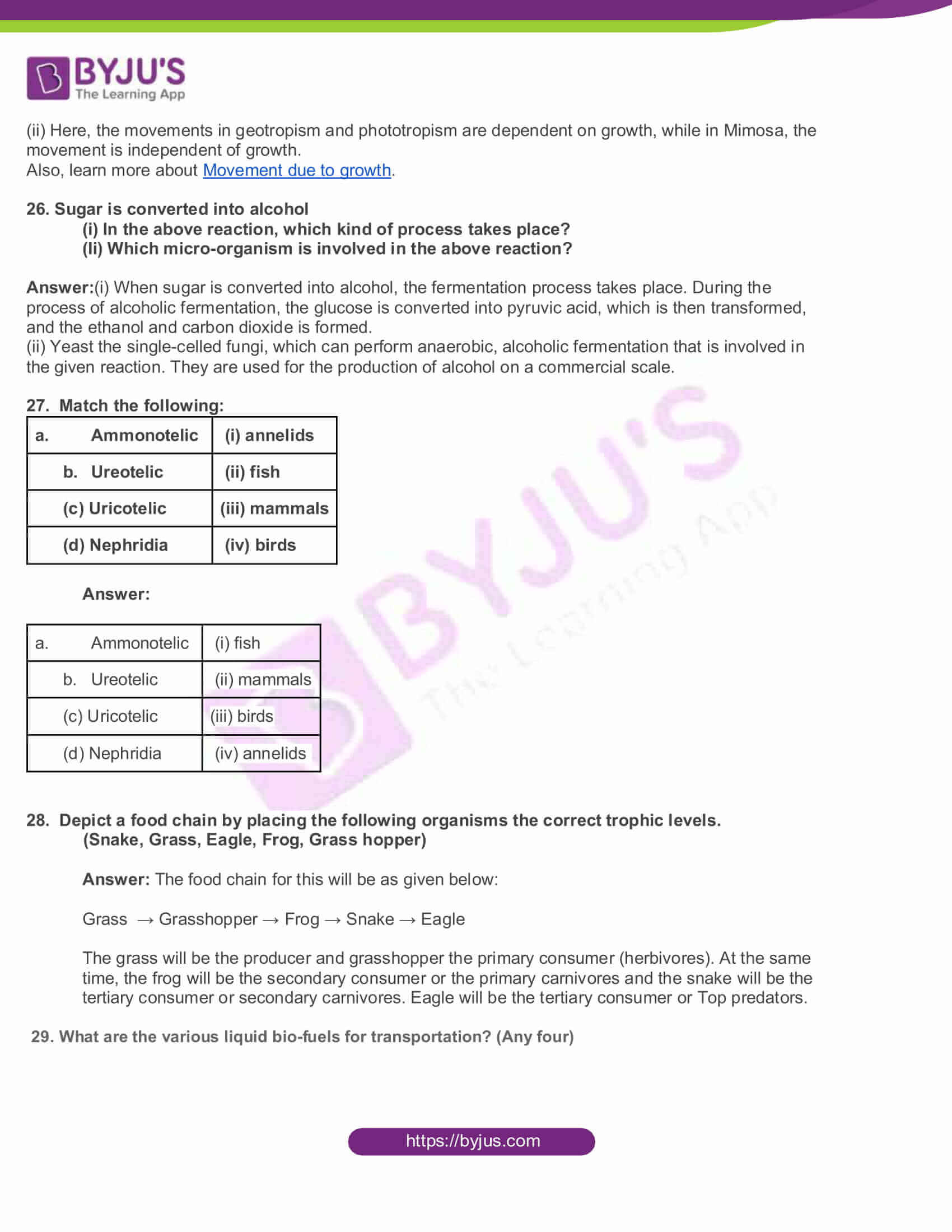
- Observe the diagram
(i) Mention the type of movements shown in figure A and B
(ii) How does this movement differ from the movement of mimosa?
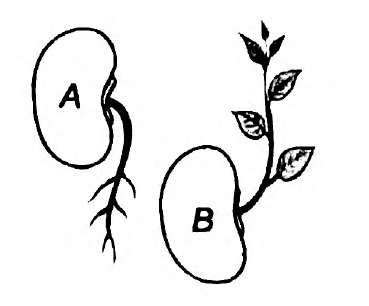
Answer: (i) Geotropism is the movement of the plant parts in response to gravity. The roots grow towards gravity and are hence called positively geotropic. The stem, on the other hand, is negatively geotropic. Here, figure A depicts Geotropism. Meanwhile, figure B is for phototropism. In phototropism, the plant grows in the direction of light. The plants have a chemical called auxin, which reacts in response to light. This causes the cells of the plants to elongate. Growth towards light is positive phototropism, while that away from the light is negative phototropism. The stem grows towards the direction of light and is called positively phototropic. However, the root is called negatively geotropic since it grows away from the light source.
(ii) Here, the movements in geotropism and phototropism are dependent on growth, while in Mimosa, the movement is independent of growth.
Also, learn more about Movement due to growth.
26. Sugar is converted into alcohol
(i) In the above reaction, which kind of process takes place?
(Ii) Which micro-organism is involved in the above reaction?
Answer:(i) When sugar is converted into alcohol, the fermentation process takes place. During the process of alcoholic fermentation, the glucose is converted into pyruvic acid, which is then transformed, and the ethanol and carbon dioxide is formed.
(ii) Yeast the single-celled fungi, which can perform anaerobic, alcoholic fermentation that is involved in the given reaction. They are used for the production of alcohol on a commercial scale.
27. Match the following:
|
(i) annelids |
|
(ii) fish |
| (c) Uricotelic | (iii) mammals |
| (d) Nephridia | (iv) birds |
Answer:
|
(i) fish |
|
(ii) mammals |
| (c) Uricotelic | (iii) birds |
| (d) Nephridia | (iv) annelids |
28. Depict a food chain by placing the following organisms the correct trophic levels.
(Snake, Grass, Eagle, Frog, Grass hopper)
Answer: The food chain for this will be as given below:
Grass → Grasshopper → Frog → Snake → Eagle
The grass will be the producer and grasshopper the primary consumer (herbivores). At the same time, the frog will be the secondary consumer or the primary carnivores and the snake will be the tertiary consumer or secondary carnivores. Eagle will be the tertiary consumer or Top predators.
29. What are the various liquid bio-fuels for transportation? (Any four)
Answer: The various liquid bio-fuels used for transportation includes bioethanol or bio alcohol, biogas, biodiesel and vegetable oils.
30. Match the renewable and non-renewable sources:
| Sources | A | B | C |
| Renewable | Coal | Wind | Petroleum |
| Non-Renewable | Hydrogen | Natural Gas | Solar Energy |
Answer:
| Sources | A | B | C |
| Renewable | Hydrogen | Wind | Solar Energy |
| Non-Renewable | Coal | Natural Gas | Petroleum |
31. Fossil fuels are formed by decomposition of biomass buried under earth over millions of years ago. Name any two fossil fuels.
Answer: Coal, crude oil and natural gas are all considered as fossil fuels.
32. What is the Brownian Movement?
Answer:The
Brownian movement also called Brownian motion is defined as the uncontrolled or erratic movement of particles in a fluid due to their constant collision with other fast-moving molecules.
33. Find the concentration of solution in terms of weight percent if 20g of common salt is dissolved in 50 g of water.
Answer: Given that weight of salt (NaCl)= 20g
And the weight of water(H2O) = 50g
Calculate weight percentage = weight of solute / (weight of solute + weight of solvent) X 100
Therefore, weight percentage = 20 /(20+50) X 100 = 28.57%
34. Calculate the number of moles in 12.046 X 1022 atoms of copper.
Answer: To calculate the number of moles in an atom, use the formula
Number of moles of atoms = Number of atoms / Avogadro’s number
So, replacing the values in the formula you get (12.046 X 1022) / 6.023 X 1023 = 2 X 1/10 = 0.2 moles
35. Two acids “A” and “B” were kept in beakers. Acid “A” undergoes partial dissociation in water, whereas acid “B” undergoes complete dissociation in water.
(i) Of the two acids “A” and “B”, which is weak acid and which is strong acid?
(ii) Give one example each for a weak and a strong acid.
Answer: (i) A weak acid is only partially dissociated, and it has both the undissociated acid and its dissociation products present, in solution, in equilibrium with each other. For this reason, acid A is the weak acid, and acid B is the strong acid.
(ii) Acetic acid (CH 3COOH) is an example of a weak acid, while HCl is a strong acid.
36. Pick the odd one out:
(i) Inorganic acids: HCl, HNO3, H2SO4, HCOOH
(ii) Basic nature: Blood, Baking soda, Vinegar, Household Ammonia
Answer: (i) HCOOH is the odd man out as it is organic acid
(ii) Odd man out is Vinegar, as it is acidic in nature
37. Correct the mistakes, if any in the following statement.
(i) Second period is a short period. It contains only 2 elements
(ii) Group 18 elements are called Halogen family
Answer: (i) Second period is a short period. It contains only 8 elements
The shortest period is the first period with only two elements.
(ii) Group 18 elements are noble gases, while Group 17 elements are called Halogen family
38. Assertion: A greenish layer appears on copper vessels, if left uncleaned.
Reason: It is due to the formation of a layer of basic copper carbonate
- Assertion and reason are correct and relevant to each other
- Assertion is true but reason is not relevant to the assertion
Answer: (a) Assertion and reason are correct and relevant to each other
39. An Organic Compound(A) is widely used as a preservative in pickle and has a molecular formula C2H4O2. This compound reacts with ethanol to form a sweet smelling compound (B).
(i) Identify the compounds A and B
(ii) Name the process and write the corresponding chemical equation
Answer: (i) The Organic compound (A) widely used as a preservative in a pickle is Vinegar- Acetic Acid(CH3COOH). When acetic acid reacts with ethanol in the presence of conc. H2SO4, it forms esters with a sweet smell.
So compound A would be Acetic Acid or Ethanoic Acid, while compound B is Ethyl Ethnoate or ester
(ii) When carboxylic acid and alcohol react, the product formed is known as an ester. Below is an example of the formation of an ester from the reaction of ethanoic acid with absolute ethanol in the presence of an acid as a catalyst.
CH3COOH + CH3CH2OH → CH3COOCH2CH3
(Ethanoic acid) (Ethanol) (Esters)
40. Assertion (A) : MRI is used to scan the inner organs of the human body by penetrating a very intense magnetic field.
Reason(R): By use of a very intense magnetic field, very high resolution images can be obtained.
(a) (A) is incorrect and (R) is correct
(b) (A) is correct (R) is incorrect
(c) Both (A) and (R) are incorrect
(d) (A) is correct and (R) supports A
Answer: (d) (A) is correct and (R) supports A
41. An object of mass 1 kg is dropped from a height of 20 m. It hits the ground and rebounds with the same speed. Find the change in momentum.
(Take g= 10 m/s2)
Answer: Take the mass of the object (m) = 1 kg
Height (h) = 20 m
Now, to calculate the velocity with which the object hits the ground replace values to the equation v1= √2gh = √2X10X20 = √400 = 20 ms-1.
Meanwhile, Velocity with which the object rebounds/ (v2) = -20 ms-1
Therefore, change in momentum = Final momentum – Initial momentum = mv2-mv1
mv2-mv1= [(1 × (–20)] – (1 × 20) = –20 – 20 = – 40 kg ms-1
Hence, magnitude of change in momentum is 40 kg ms-1
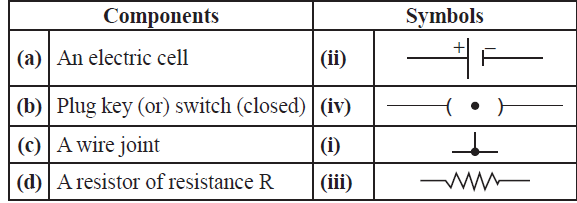
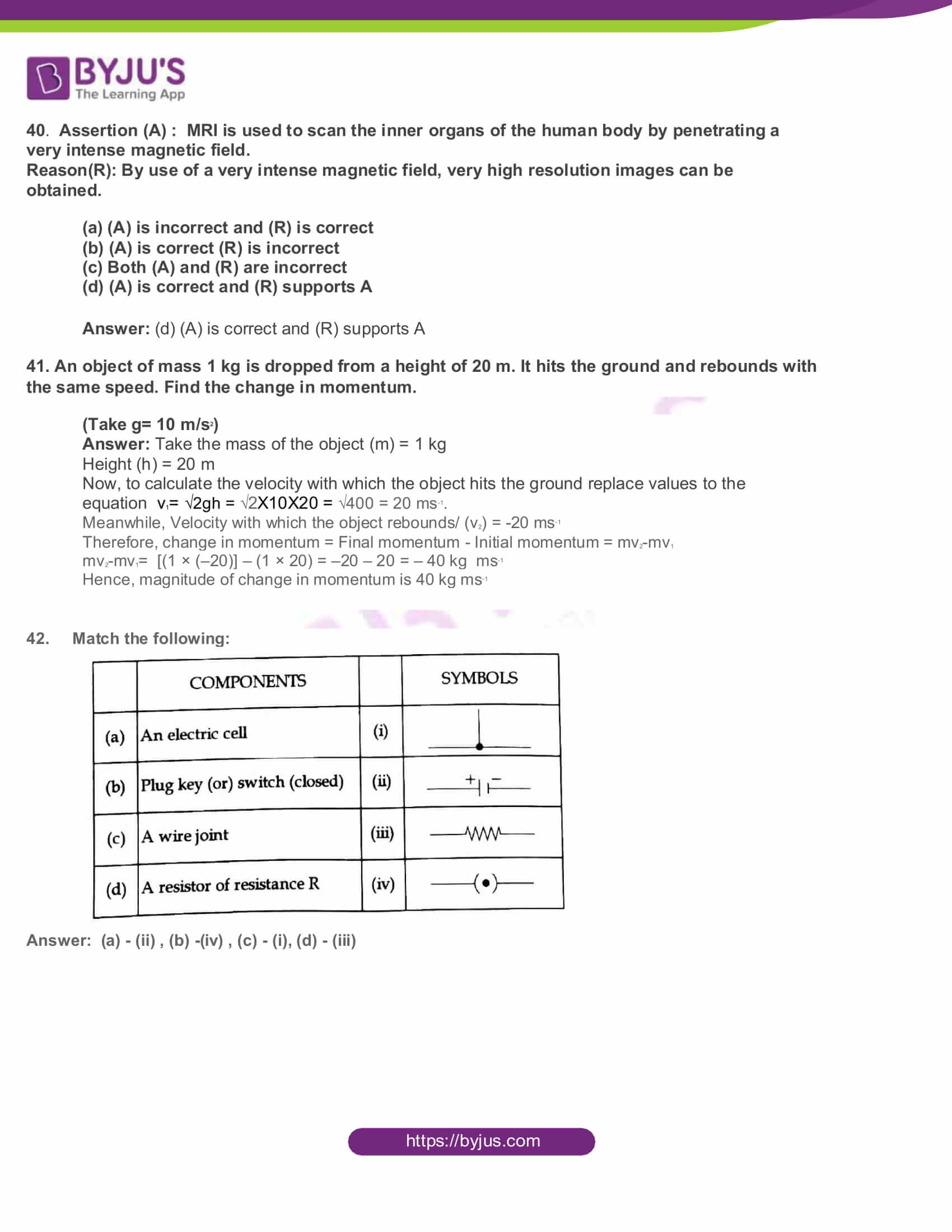
42. Match the following:
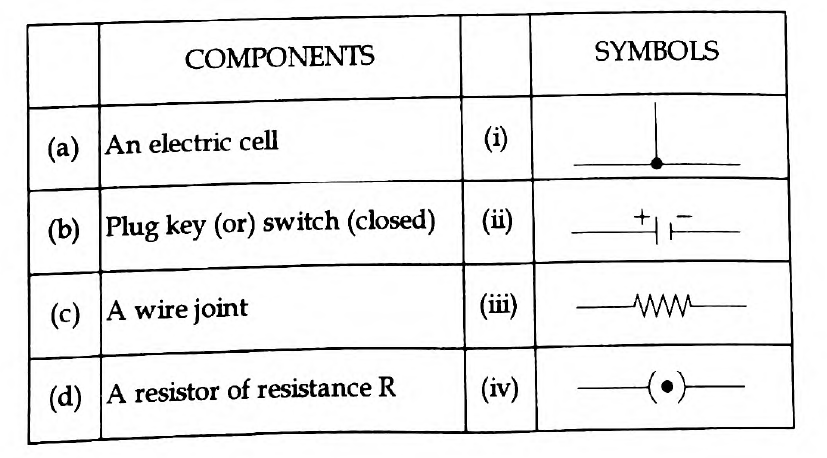
Answer: (a) – (ii) , (b) -(iv) , (c) – (i), (d) – (iii)
43. Fill in the blanks:
(i) Potential Difference: Voltmeter ; then Current: _______
(ii) Hydro power plant : Conventional Source of Energy ; then Solar Energy : ______
Answer: (i) Ammeter
(ii) Non–conventional source of energy
44. Write about ocean thermal energy.
Answer: Ocean Thermal Energy also called Ocean Thermal Energy Conversion (OTEC) refers to a method of using the temperature difference between the deep parts of the sea which are cold and the shallow parts of the sea which are cold to run a heat engine and produce useful work.
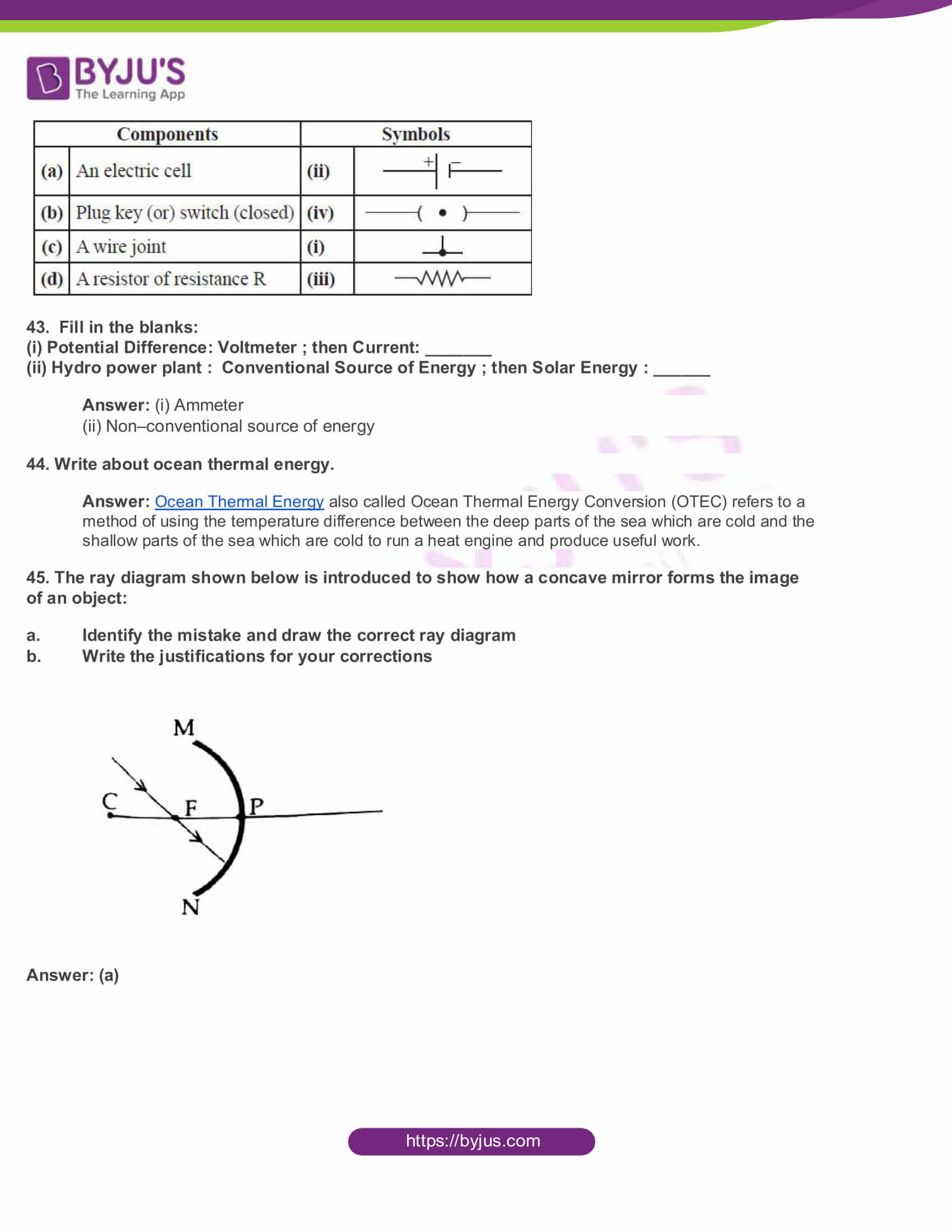
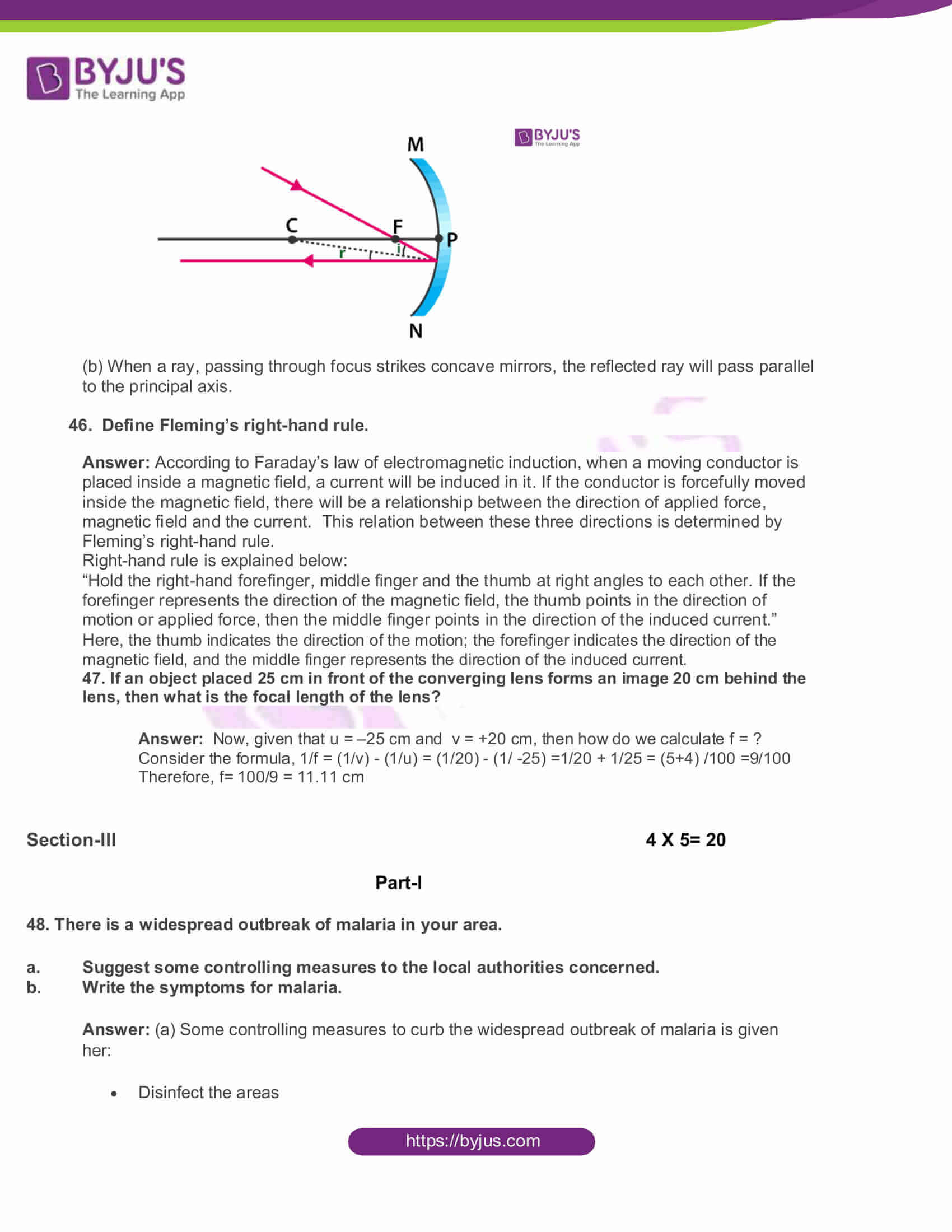
45. The ray diagram shown below is introduced to show how a concave mirror forms the image of an object:
- Identify the mistake and draw the correct ray diagram
- Write the justifications for your corrections
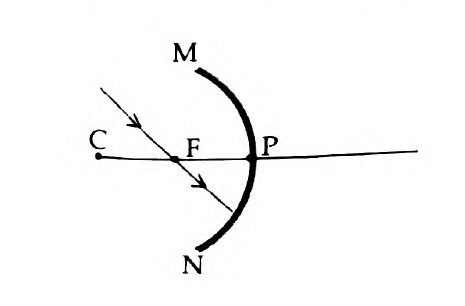
Answer: (a)
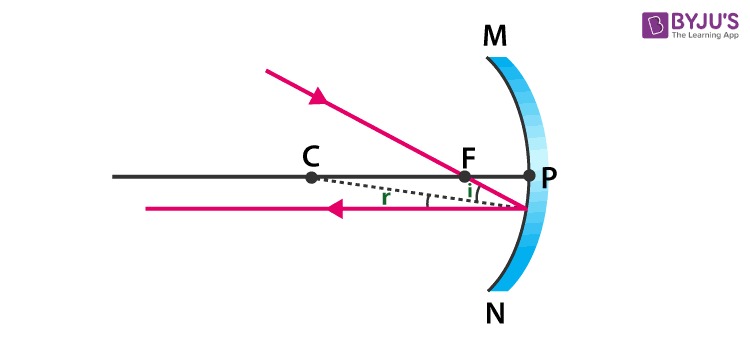
(b) When a ray, passing through focus strikes concave mirrors, the reflected ray will pass parallel to the principal axis.
46. Define Fleming’s right-hand rule.
Answer: According to Faraday’s law of electromagnetic induction, when a moving conductor is placed inside a magnetic field, a current will be induced in it. If the conductor is forcefully moved inside the magnetic field, there will be a relationship between the direction of applied force, magnetic field and the current. This relation between these three directions is determined by Fleming’s right-hand rule.
Right-hand rule is explained below:
“Hold the right-hand forefinger, middle finger and the thumb at right angles to each other. If the forefinger represents the direction of the magnetic field, the thumb points in the direction of motion or applied force, then the middle finger points in the direction of the induced current.”
Here, the thumb indicates the direction of the motion; the forefinger indicates the direction of the magnetic field, and the middle finger represents the direction of the induced current.
47. If an object placed 25 cm in front of the converging lens forms an image 20 cm behind the lens, then what is the focal length of the lens?
Answer: Now, given that u = –25 cm and v = +20 cm, then how do we calculate f = ?
Consider the formula, 1/f = (1/v) – (1/u) = (1/20) – (1/ -25) =1/20 + 1/25 = (5+4) /100 =9/100
Therefore, f= 100/9 = 11.11 cm
Section-III 4 X 5= 20
Part-I
48. There is a widespread outbreak of malaria in your area.
- Suggest some controlling measures to the local authorities concerned.
- Write the symptoms for malaria.
Answer: (a) Some controlling measures to curb the widespread outbreak of malaria is given her:
- Disinfect the areas
- Prevent water stagnation and keep the ditches and drains covered
- Use mosquito nets and repellents
(b) Some symptoms of malaria includes chills, shivering and a feverish feeling
49. Use the words from the given list to complete the following paragraph:
(Vertebral column, Piamater, Arachnoid membrane, Meninges, Duramater)
- The central nervous system is covered by three protective coverings collectively called ___(1)___ . The outermost cover lying below the skull and ___(2)___ is double thick and is called __(3)___. The middle covering is thin and vascularised and is called__ (4)__ . The innermost cover is a very thin delicate membrane and is closely stretched over the outer surface of Brain and Spinal Cord and is called___(5)_____
(b) Name five types of nerve cells______
Answer: (a) (1) Meninges
(2) Vertebral column
(3) Durameter
(4) Arachnoid membrane
(5) Piamater
- Below given are the types of nerve-cells:
(1) Myelinated or Medullated or White neurons.
(2) Non- Myelinated or Non-Medullated or Grey neurons.
(3) Unipolar neurons.
(4) Bipolar neurons.
(5) Multipolar neuron.
Part-II
50. Describe the structure of a dicot seed with a neat diagram.
Answer:
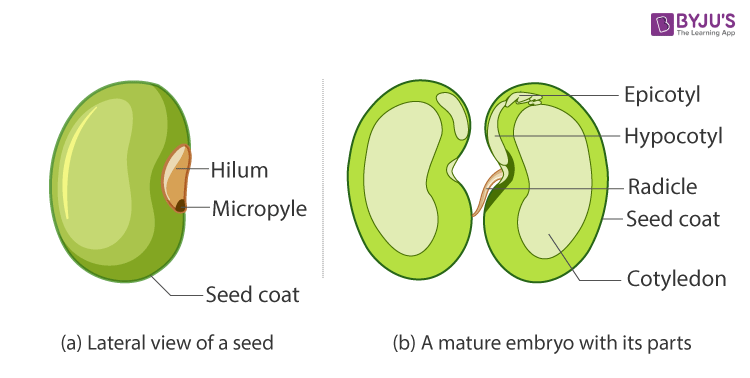
Structure of a Dicotyledonous Seed
- Peas, almonds and cashews are examples of dicotyledons or dicot seeds.
- Dicotyledons are also known as dicots. They are the groups into which all the flowering plants or angiosperms were formerly divided. The name dicotyledons refer to the seed having two embryonic cotyledons. There are around 200,000 species of dicotyledons discovered till date.
- In dicotyledons, the embryo consists of an embryo axis and two cotyledons. Cotyledons generally have a swollen appearance as it acts as a food reserve for the developing seedling. The embryo axis has two ends. The one which forms the shoot tip is called plumule and the portion at the lower end which forms the root tip is called the radicle. The whole content is enclosed within a protective cover called the seed coat. The seed coat is made up of an outer layer called testa and an inner layer called tegmen. Moreover, the seed is attached to the fruit through a structure called hilum.
- Other dicot seeds examples include apples, plums and peaches.
51. (a) What is Green Chemistry?
(b) What is the future products of Green Chemistry?
Answer: (a) Green Chemistry also referred to as Sustainable Chemistry is the branch of Chemistry that deals with the design and optimisation of processes and products in order to lower or remove altogether, the production and use of toxic substances. Green chemistry is not the same as environmental chemistry. The primary focus of the former is on the environmental impact of chemistry and the development of sustainable practices that are environment-friendly (such as a reduction in the consumption of non-renewable resources and strategies to control environmental pollution). Meanwhile, the latter focuses on the effects that certain toxic or hazardous chemicals have on the environment.
(b) Future products:
- Incorporation of renewable feedstock: the use of renewable feedstock and renewable raw materials must be preferred over the use of non-renewable ones.
- Energy efficiency: The amount of energy consumed by the process must be minimized to the maximum possible extent.
- Prevention of waste: preventing the formation of waste products is always preferable to the clean-up of the waste once it is generated.
Part- III
52. Modern atomic theory takes up the wave concept, principle of uncertainty and other latest discoveries to give a clear cut picture about an atom. State the findings of modern atomic theory
Answer: See the findings of the atomic theory from here.
53. Explain the manufacturing of Ethanol from molasses.
Answer: A dark syrupy liquid left after the crystallisation of sugar from the concentrated sugar cane juice is molasses. Molasses still contains sucrose, which is converted to ethanol. See the steps below:
(i) First step is Distillation. Molasses is distilled with water to cut down the concentration of sugar to 8% to 10%.
(ii) Secondly, add ammonium salts. Usually, molasses have enough nitrogenous matter that can act as food for yeast, during the fermentation process. However, if the nitrogen content in molasses is few, it can be fortified by adding ammonium phosphate or ammonium sulphate.
(iii). Now, add yeast. The solution from step (ii) is then collected in large ‘fermentation tanks’, and yeast is added to it. The mixture is kept at about 303K for a few days. During this period, the enzymes invertase and zymase present in yeast, cause the conversion of sucrose into ethanol.
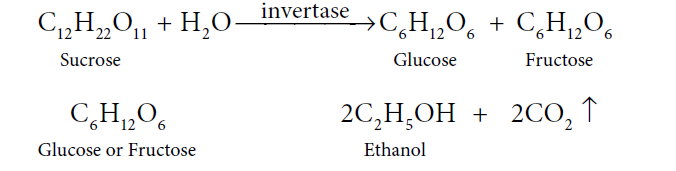
Now, here the fermented liquid is technically known as wash.
(iv) The final step is the distillation of wash. The fermented liquid with 15% to 18% alcohol and the rest of the water, is now subjected to fractional distillation. The main fraction drawn is an aqueous solution of ethanol that contains 95.5% of ethanol and 4.5 % of water, called rectified spirit. The mixture is then heated under reflux over quicklime for about 5 to 6 hours and then allowed to stand for 12 hours. On distillation of this mixture, pure alcohol (100%) called absolute alcohol is obtained.
Part-IV
54. 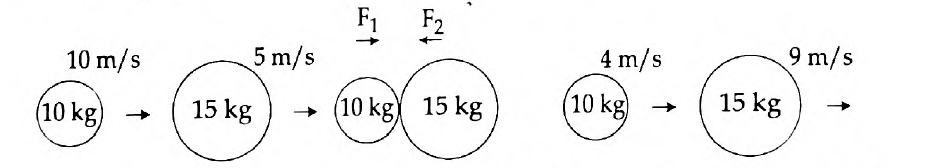
- Newton’s first law of motion gives a qualitative definition of force. Justify.
- The figure represents two bodies of masses 10 kg and 15 kg, moving with an initial velocity of 10 ms-1 and 5 ms-1 respectively. They collide with each other. After collision, they move with velocities 4 ms-1 and 9 ms-1 respectively. The time of collision is 2 s. Now, calculate F1 and F2.
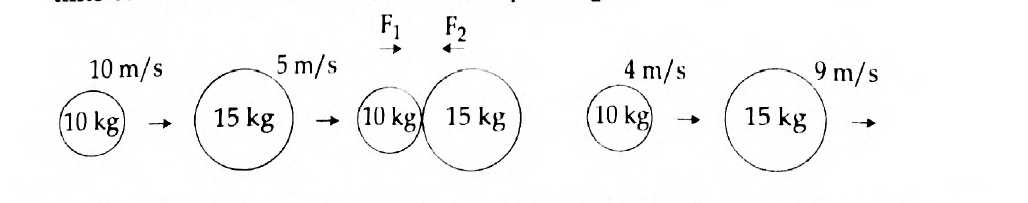
Answer: (a) Newton’s First Law of Motion states that a body remains in the state of rest or uniform motion in a straight line unless and until an external force acts on it. It is said that according to the first law, a qualitative definition is given to force. See the example to justify this:
Wearing a seat belt in a car while driving is an example of Newton’s 1st law of motion. If an accident occurs, or if brakes are applied to the car suddenly, the body will tend to continue its inertia and move forward, probably proving fatal. To prevent such accidents seat belts are used, which stops your body from moving forward in inertia, thus avoiding danger.
(b) Given here is the Mass of a body A, (m1) = 10 kg
Mass of a body B, (m2) = 15 kg
Initial velocity of A, (u1) = 10 m/s
Initial velocity of B, (u2) = 5 m/s
Final velocity of A, (v1) = 4 m/s
Final velocity of B, (v2) = 9 m/s and
The time of collision, (t) = 2 seconds.
Applying Newton’s second law of motion, Force acting on B (action),
F1 = mass of B × acceleration on B
Therefore, F1 = m2(v2-u2) /t = 15(9-5)/2 = 15 × 4 / 2= 30 N
Meanwhile, Force acting on A (reaction), F2 = mass of A × acceleration on A
That is, F2 = m1(v1-u1) /t = 10(4-10) / 2 = 10 × (-6) / 2 = -30 N
Now, according to Newton’s third law of motions F1 = – F2
Hence, F1 = 30 N
F2 = -30 N
55. State and explain the defects of vision. How can these defects be rectified?
Answer: Some defects of vision include myopia, hyperopia or hypermetropia and presbyopia. Learn about these defects and how to rectify them.







Comments