Ever wondered why your neck region gets swollen when you are down with cold and flu? This is because WBCs accumulate around that region, which is our bodies’ defence mechanism in the blood.
Table of Contents
- What Are Antibodies
- Antibody Structure
- Types Of Antibodies
- Functions Of Antibody
- Production And Mechanism Of Antibody
- Difference-Between-Antigen-And-Antibody
- Frequently Asked Questions
Antibodies are not found at a place as such, but whenever our immune system encounters antigen or a pathogen, B cells get activated immediately releasing antibodies into the bloodstream. These immunoglobulins undergo mitosis resulting in cell division and continuously produce antibodies as a result of producing more cells. These antibodies remain in the blood for some time but B cells remember these antigens and repeat the same course of action whenever they reappear in our body.
What are Antibodies?
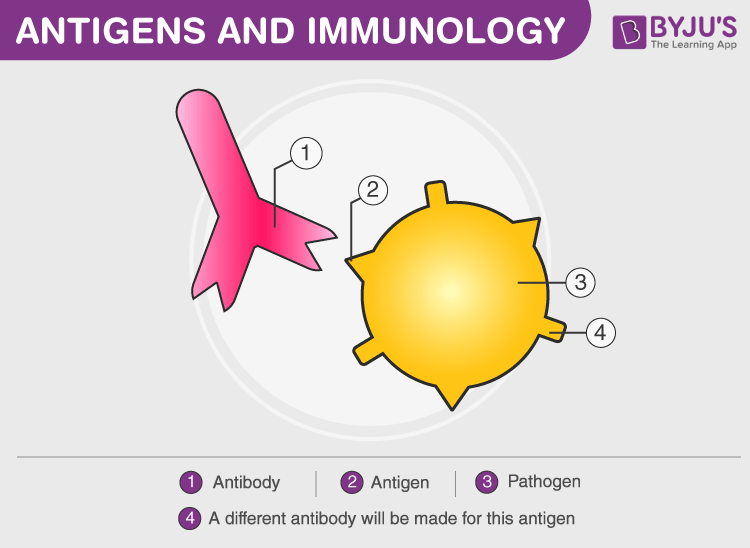
Antibody (Ab) is also known as an immunoglobulin(Ig). These are large, Y-shaped blood proteins produced by plasma cells. They bind to foreign particles and invade them. These particles are foreign bodies that get attacked by Antibody.
Antigens are foreign pathogens that invade the body and have the capability to give rise to a response from our immunity system either by grouping up with a larger molecule or alone after binding with antibodies for a particular immune response. Hence, antigens stimulate the production of antibodies by the immune system.
Antibody Structure
An antibody has a Y-shaped structure, made up of four polypeptide subunits. Each subunit has two identical light and heavy chains.
The N-terminus of each heavy chain forms an antigen-binding domain with a light chain. There are two antigen-binding domains forming the arms of the “Y” shape. They are known as ‘fragment antigen-binding’ (Fab) domains.
The C-terminus of the heavy chains forms ‘fragment crystallization’ (Fc) domain, which helps in the interaction with the effector cells.
All four polypeptide subunits are held together by disulfide and non-covalent bonds.
The heavy chains of the antibodies contain a variable region and three constant regions. Each antibody has two identical antigen-binding sites and they differ in the antibodies.
Also Read: Difference between B cells and T cells
Types Of Antibodies
Antibodies or immunoglobulins(Ig) are of five different isotypes. This classification is on the basis of their H chains. Let’s look at the different types of immunoglobulins and their functions.
IgM
IgM is the first antibody produced in response to a microbial attack by B cells.
It is the largest antibody and is found in a pentameric form.
It circulates in the blood and lymph and constitutes 6% of the total antibody content in the serum.
It is involved in agglutination and opsonization.
It has a large number of antigenic sites on its surface and therefore facilitates efficient activation of the immune system.
IgG
Most abundant isotype in the plasma, and comprises 80% of the total antibody content in the serum. It detoxifies substances that are harmful and recognizes the antibody-antigen complex.
It is transferred to the placenta through the foetus and protects the infant until its birth.
IgG is divided into four subclasses- IgG1, IgG2, IgG3, and IgG4. Among these, only IgG3 and IgG4 possess the ability to cross the placenta.
The heavy chains of IgG have two antigen-binding sites and are of the sub-class gamma.
It facilitates the process of phagocytosis and provides immunity to the developing foetus. It neutralizes the toxins and pathogens and offers protection to the body.
IgA
Usually found in liquids such as breast milk, serum, saliva, fluids of the intestine. IgA in breast milk protects an infant’s gastrointestinal tract from microbial activity.
It constitutes 13% of the total antibody content in the serum and is divided into 2 sub-classes- IgA1 and IgA2. Among these, IgA1 is highly found in the secretions and is also called the secretory immunoglobulin.
It exists in both monomeric as well as dimeric forms.
It provides the first line of defence against the pathogens and limits inflammation. It also activates the complement pathway and participates in the immune response.
IgD
It is involved in the production of the antibody by B cells.
It is present as a monomer and weighs around 1,80,000 dalton.
It comprises less than 1% of the total antibody content in serum.
It acts as a receptor on B cell surface and participates in B cell activation and differentiation.
IgE
IgE is present in the least amounts, around 0.02% of the antibody content in the serum.
These are present in the linings of the respiratory and intestinal tracts and respond to allergic reactions.
This is found as a monomer in the body and weighs about 200,000 Dalton.
Also Read: Difference between active and passive immunity
Functions of Antibody
Following are some of the key functions of antibody:
-
Binds to pathogens
-
Activates the immune system in case of bacterial pathogens
-
Directly attacks viral pathogens
-
Assists in phagocytosis
-
Antibody provides long-term protection against pathogens because it persists for years after the presence of the antigen.
-
It neutralizes the bacterial toxins and binds the antigen to enhance its efficiency.
-
They also act as the first line of defence for mucosal surfaces.
-
They ingest cells by phagocytosis.
-
Few antibodies bind the antigen present on the pathogens. These aggregates the pathogen and they remain in secretions. When the secretion is expelled out, the antigen is also expelled.
Production And Mechanism Of Antibody
Whenever an organism’s immune system encounters a foreign particle for the first time, macrophages interfere and capture to break them down so as to pass them to B cells. Once these antigens are presented, B cells begin production of a new antibody which would contain a unique paratope (site at which antibody binds with antigen) to bind with a specific epitope (site in the antigen that binds with antibody). Each lymphocyte of B cells generates a unique antibody against a unique epitope. Once the encoding is done by B cells, it releases antibodies which then bind with specific pathogens resulting in their elimination from our bodies.
This is achieved either by direct attack of antibody on pathogens (usually when pathogens are viruses) or by binding to pathogen’s surface (when the pathogen is a bacteria) and sending signals to the rest of the immune system to eliminate the pathogen. These cells remain in the body forever, ready to attack, should they re-enter the body.
Difference Between Antigen And Antibody
|
Antigen |
Antibody |
| These molecules interact with antibodies or by T-cell receptors when complexed with major histocompatibility complex | Synthesized by plasma cells of B cells that react with antigens who invoked their production |
| Includes components of viral proteins, cell walls, capsules, and other microbes | Consists of 4 polypeptide chains, two light chains(L chain) and two heavy chains(H chain) forming a Y shape |
| These are proteins but can be nucleic acids, carbohydrates and lipids | These are glycoproteins made up of carbohydrates and amino acids |
| Highly complex in structure and composition | Simpler in structure |
| Causes diseases or allergic reactions | Protects the body against diseases |
| The pathogen has many epitopes. Epitopes are regions on antigens that interact with antibodies | The region of an antibody that binds with an epitope is called a paratope. Typically, a Y shaped antibody has 2 identical paratopes |
The article gives a detailed account of antibody including antibody structure, types of antibodies, functions of antibody, and its production. It also explains how an antibody is different from an antigen.
For more information on Antibody-Role of Antibody, keep visiting BYJU’S website or download BYJU’S app for further reference.
Related Links: Antigens and Immunology

Comments