The emission and absorption spectra difference is provided here. These two topics are one of the most interesting concepts in Physics. Learn their differences in detail, given in the table provided below.
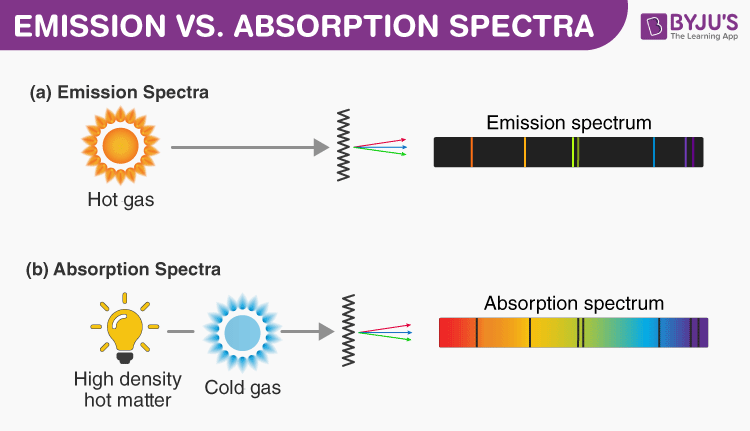
What is an Emission Spectrum?
When energy is absorbed by electrons of an atom, electrons move from lower energy levels to higher energy levels. These excited electrons have to radiate energy to return to ground states from the excited state, which is unstable. The emission spectrum is formed by the frequencies of this emitted light.
What is the Absorption Spectrum?
On the other hand, an absorption spectrum is constituted by the frequencies of light transmitted with dark bands when energy is absorbed by the electrons in the ground state to reach higher energy states.
Emission Spectra VS Absorption Spectra
The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum, whereas an absorption spectrum has dark-coloured lines in the spectrum. More differences between absorption and emission spectrum are given below in a tabular column.
| Emission Spectra | Absorption Spectra |
| Produced when atoms release energy | Produced when atoms absorb energy |
| Comprise coloured lines in the spectrum | Comprise dark lines or gaps in the spectrum |
| It is helpful in figuring out the composition of a certain matter | Can be used to figure out the ability of certain objects to retain heat and its absorption level |
| The type of photons emitted is helpful in figuring out the kind of elements the substance is made of as each element radiates a different amount of energy and has a unique emission level | The wavelengths of light absorbed is helpful in figuring out the number of substances in the sample |
Emission spectra can emit all the colours in an electromagnetic spectrum, while the absorption spectrum can have a few colours missing due to the redirection of absorbed photons.
Read More: Emission Spectrum
Recommended Video

Frequently Asked Questions – FAQs
What is the emission spectrum?
What is the absorption spectrum?
How does electromagnetic radiation occur?
What is the electromagnetic spectrum?
Define wavelength.
To know more differences like the difference between RTD and thermocouple, visit BYJU’S.


Comments